Characterization of a Novel Thermostable DNA Lyase Used To Prepare DNA for Next-Generation Sequencing
- PMID: 36647573
- PMCID: PMC9945173
- DOI: 10.1021/acs.chemrestox.2c00172
Characterization of a Novel Thermostable DNA Lyase Used To Prepare DNA for Next-Generation Sequencing
Abstract
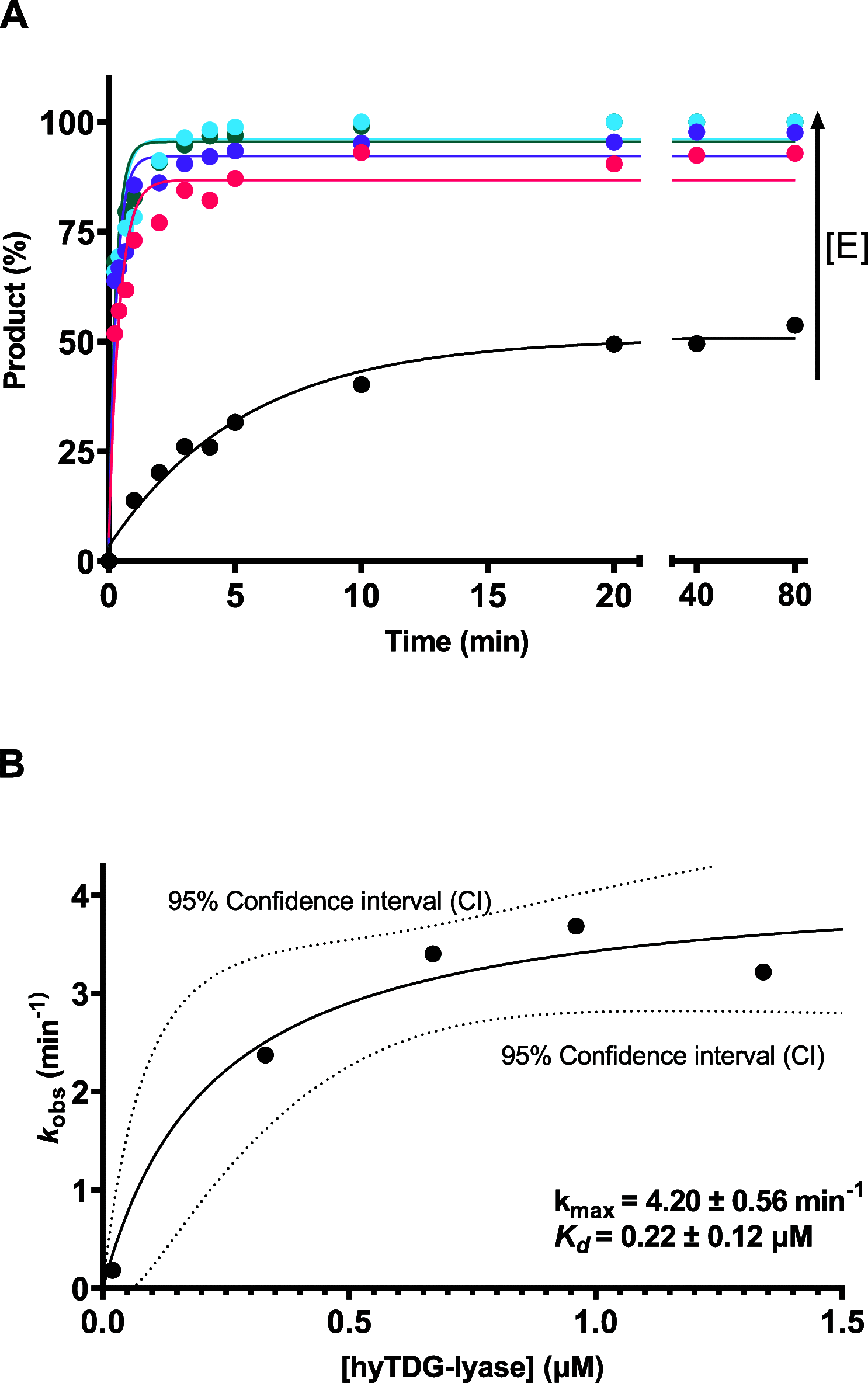
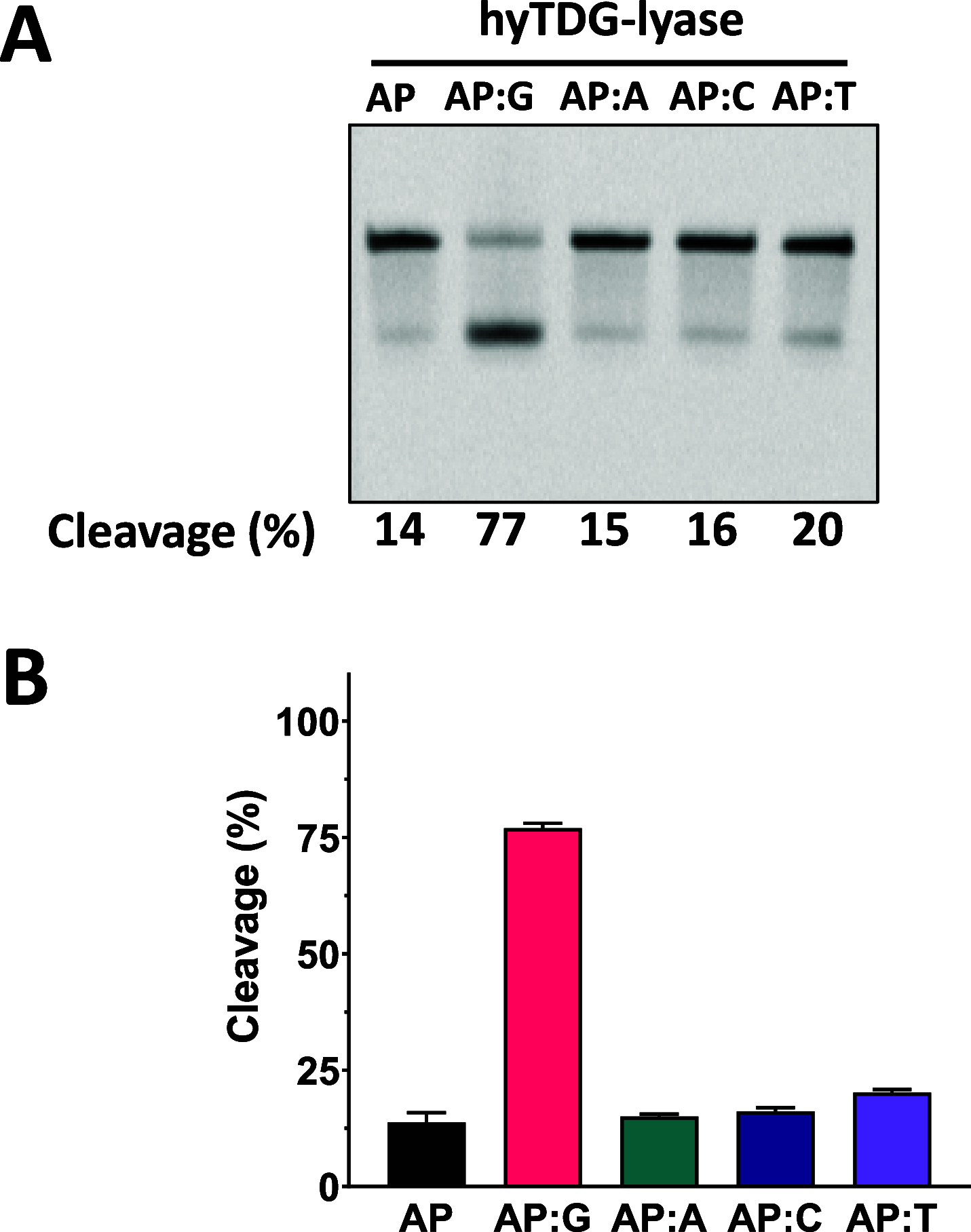
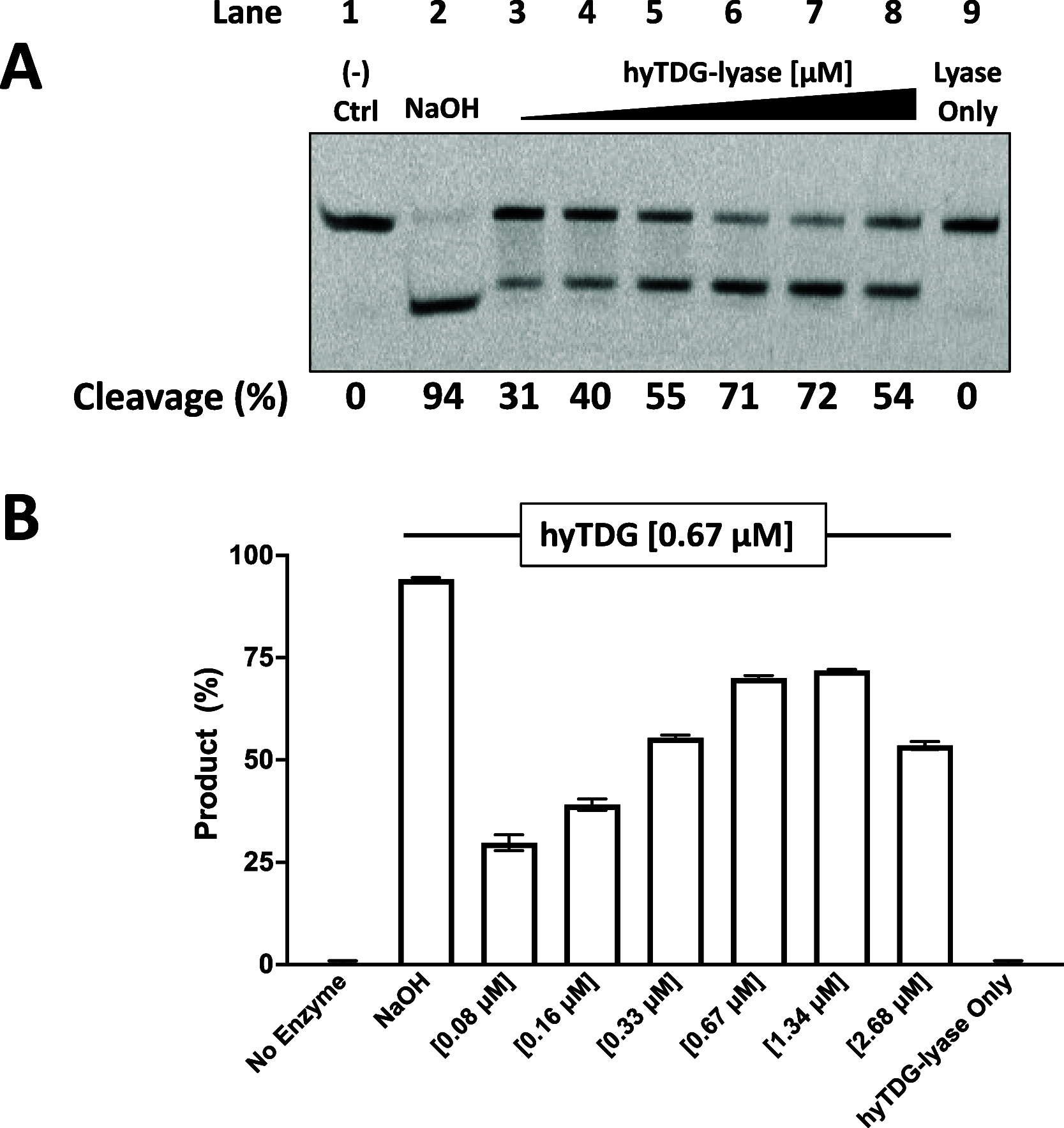
Recently, we constructed a hybrid thymine DNA glycosylase (hyTDG) by linking a 29-amino acid sequence from the human thymine DNA glycosylase with the catalytic domain of DNA mismatch glycosylase (MIG) from M. thermoautotrophicum, increasing the overall activity of the glycosylase. Previously, it was shown that a tyrosine to lysine (Y126K) mutation in the catalytic site of MIG could convert the glycosylase activity to a lyase activity. We made the corresponding mutation to our hyTDG to create a hyTDG-lyase (Y163K). Here, we report that the hybrid mutant has robust lyase activity, has activity over a broad temperature range, and is active under multiple buffer conditions. The hyTDG-lyase cleaves an abasic site similar to endonuclease III (Endo III). In the presence of β-mercaptoethanol (β-ME), the abasic site unsaturated aldehyde forms a β-ME adduct. The hyTDG-lyase maintains its preference for cleaving opposite G, as with the hyTDG glycosylase, and the hyTDG-lyase and hyTDG glycosylase can function in tandem to cleave T:G mismatches. The hyTDG-lyase described here should be a valuable tool in studies examining DNA damage and repair. Future studies will utilize these enzymes to quantify T:G mispairs in cells, tissues, and genomic DNA using next-generation sequencing.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors have submitted a patent application for hyTDG and hyTDG-lyase. Otherwise, the authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Zhao S.; Tadesse S.; Kidane D.. Significance of Base Excision Repair to Human Health, 1st ed.; Elsevier Inc., 2021; Vol. 364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

