Safety and efficacy of tumour-treating fields (TTFields) therapy for newly diagnosed glioblastoma in Japanese patients using the Novo-TTF System: a prospective post-approval study
- PMID: 36647599
- PMCID: PMC10150168
- DOI: 10.1093/jjco/hyad001
Safety and efficacy of tumour-treating fields (TTFields) therapy for newly diagnosed glioblastoma in Japanese patients using the Novo-TTF System: a prospective post-approval study
Erratum in
-
Correction to: Safety and efficacy of Tumor Treating fields (TTFields) therapy for newly diagnosed glioblastoma in Japanese patients using the Novo-TTF System: a prospective post-approval study.Jpn J Clin Oncol. 2023 Mar 30;53(4):370. doi: 10.1093/jjco/hyad014. Jpn J Clin Oncol. 2023. PMID: 36806850 Free PMC article. No abstract available.
Abstract
Background: Tumour-treating fields therapy is a locoregional, anti-cancer treatment. Efficacy and safety of tumour-treating fields therapy in adults with newly diagnosed glioblastoma were demonstrated in the pivotal phase 3 EF-14 study (NCT00916409). Here, we report post-approval data of tumour-treating fields therapy in Japanese patients with newly diagnosed glioblastoma.
Methods: Unsolicited post-marketing surveillance data from Japanese patients with newly diagnosed glioblastoma treated with tumour-treating fields therapy (December 2016-June 2020) were retrospectively analysed. The primary endpoints were skin, neurological and psychiatric adverse events. The secondary endpoints were 1- and 2-year overall survival rates, and the 6-month progression-free survival. adverse events were analysed using MedDRA v24.0. The overall survival and progression-free survival were assessed using the Kaplan-Meier survival analysis (log-rank testing). The Cox proportional hazard regression analyses were also performed.
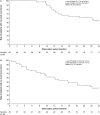
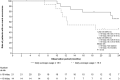
Results: Forty patients with newly diagnosed glioblastoma were enrolled (62.5% male; median age 59 years; median baseline Karnofsky Performance Scale score 90). The most common tumour-treating-fields-therapy-related adverse event was beneath-array local skin reaction (60% of patients). The adverse events were mostly mild to moderate in severity. Neurological disorders were observed in 2.5% patients (one patient reported dysesthesia). No psychiatric disorders were reported. The 1- and 2-year overall survival rates were 77.9% (95% CI 60.6-88.3) and 53.6% (35.5-68.7%), respectively. The 6-month progression-free survival was 77.5% (61.2-87.6%). These survival rates compare favourably with those in the EF-14 trial (1- and 2-year overall survival rates: 73% [69-77%] and 43% [39-48%], respectively; 6-month progression-free survival rate: 56% (51-61%).
Conclusion: This post-approval, real-world evidence study revealed no new safety signals and suggests the safety and efficacy of tumour-treating fields therapy in Japanese patients with newly diagnosed glioblastoma.
Keywords: CNS; interventional therapy; new technology/instruments; skin.
© The Author(s) 2023. Published by Oxford University Press.
Figures
Similar articles
-
Tumor treating fields plus temozolomide for newly diagnosed glioblastoma: a sub-group analysis of Korean patients in the EF-14 phase 3 trial.J Neurooncol. 2020 Feb;146(3):399-406. doi: 10.1007/s11060-019-03361-2. Epub 2020 Feb 4. J Neurooncol. 2020. PMID: 32020470 Clinical Trial.
-
Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial.JAMA Oncol. 2018 Apr 1;4(4):495-504. doi: 10.1001/jamaoncol.2017.5082. JAMA Oncol. 2018. PMID: 29392280 Free PMC article. Clinical Trial.
-
Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial.CNS Oncol. 2017 Jul;6(3):185-193. doi: 10.2217/cns-2016-0049. Epub 2017 Apr 12. CNS Oncol. 2017. PMID: 28399638 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Treating Glioblastoma With Tumor-Treating Fields Therapy.Clin J Oncol Nurs. 2016 Oct 1;20(5 Suppl):S9-S13. doi: 10.1188/16.CJON.S1.9-13. Clin J Oncol Nurs. 2016. PMID: 27668388 Review.
-
Prevention and Management of Dermatologic Adverse Events Associated With Tumor Treating Fields in Patients With Glioblastoma.Front Oncol. 2020 Jul 28;10:1045. doi: 10.3389/fonc.2020.01045. eCollection 2020. Front Oncol. 2020. PMID: 32850308 Free PMC article. Review.
Cited by
-
Limited survival benefit in patients diagnosed with glioblastoma post-2016: a SEER population based registry analysis.J Cancer Res Clin Oncol. 2025 Jun 2;151(6):179. doi: 10.1007/s00432-025-06171-4. J Cancer Res Clin Oncol. 2025. PMID: 40455099 Free PMC article.
-
Global post‑marketing safety surveillance of Tumor Treating Fields (TTFields) therapy in over 25,000 patients with CNS malignancies treated between 2011-2022.J Neurooncol. 2024 Aug;169(1):25-38. doi: 10.1007/s11060-024-04682-7. Epub 2024 Jun 29. J Neurooncol. 2024. PMID: 38949692 Free PMC article.
-
Efficacy of tumour-treating fields therapy in recurrent glioblastoma: A narrative review of current evidence.Medicine (Baltimore). 2023 Dec 1;102(48):e36421. doi: 10.1097/MD.0000000000036421. Medicine (Baltimore). 2023. PMID: 38050252 Free PMC article. Review.
-
Advancement in tumor treating fields of mechanism, clinical applications, and future directions.Discov Oncol. 2025 Jun 10;16(1):1049. doi: 10.1007/s12672-025-02861-0. Discov Oncol. 2025. PMID: 40495024 Free PMC article. Review.
-
Association of Tumor Treating Fields (TTFields) therapy with survival in newly diagnosed glioblastoma: a systematic review and meta-analysis.J Neurooncol. 2023 Aug;164(1):1-9. doi: 10.1007/s11060-023-04348-w. Epub 2023 Jul 26. J Neurooncol. 2023. PMID: 37493865 Free PMC article.
References
-
- The Japan Society for Neuro-Oncology . Adult glioblastoma (GBM) guidelines - CQ6 how to treat adult recurrent glioblastoma? Kanehara-shuppan, Tokyo, Japan. 2016. https://www.jsn-o.com/guideline3/CQ/006.html.
-
- Novocure . Optune®: instructions for use. 2019. https://www.optune.com/Content/pdfs/Optune_IFU_8.5x11.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical