Ironing out macrophages in atherosclerosis
- PMID: 36647723
- PMCID: PMC10157607
- DOI: 10.3724/abbs.2022196
Ironing out macrophages in atherosclerosis
Abstract
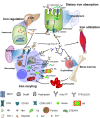
The most common cause of death worldwide is atherosclerosis and related cardiovascular disorders. Macrophages are important players in the pathogenesis of atherosclerosis and perform critical functions in iron homeostasis due to recycling iron by phagocytosis of senescent red blood cells and regulating iron availability in the tissue microenvironment. With the growth of research on the "iron hypothesis" of atherosclerosis, macrophage iron has gradually become a hotspot in the refined iron hypothesis. Macrophages with the M1, M2, M(Hb), Mox, and other phenotypes have been defined with different iron-handling capabilities related to the immune function and immunometabolism of macrophages, which influence the progression of atherosclerosis. In this review, we focus on macrophage iron and its effects on the development of atherosclerosis. We also cover the contradictory discoveries and propose a possible explanation. Finally, pharmaceutical modulation of macrophage iron is discussed as a promising target for atherosclerosis therapy.
Keywords: atherosclerosis; iron; macrophage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, et al. Atherosclerosis. Nat Rev Dis Primers. . 2019;5:56. doi: 10.1038/s41572-019-0106-z. - DOI - PubMed
-
- Engelen SE, Robinson AJB, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. . 2022;19:522–542. doi: 10.1038/s41569-021-00668-4. - DOI - PMC - PubMed
-
- Moore KJ, Koplev S, Fisher EA, Tabas I, Björkegren JLM, Doran AC, Kovacic JC. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (part 2) J Am Coll Cardiol. . 2018;72:2181–2197. doi: 10.1016/j.jacc.2018.08.2147. - DOI - PMC - PubMed
-
- Wade J, Byrne DJ, Ballentine CJ, Drakesmith H. Temporal variation of planetary iron as a driver of evolution. Proc Natl Acad Sci USA. . 2021;118:e2109865118. doi: 10.1073/pnas.2109865118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

