Substitution of gp120 C4 region compensates for V3 loss-of-fitness mutations in HIV-1 CRF01_AE co-receptor switching
- PMID: 36647730
- PMCID: PMC9980400
- DOI: 10.1080/22221751.2023.2169196
Substitution of gp120 C4 region compensates for V3 loss-of-fitness mutations in HIV-1 CRF01_AE co-receptor switching
Abstract
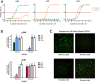
HIV-1 infection is mediated by a viral envelope subsequently binding to CD4 receptor and two main coreceptors, CCR5 (R5) for primary infection and CXCR4 (X4) in chronic infection. Switching from R5 to X4 tropism in HIV-1 infection is associated with increased viral pathogenesis and disease progression. The coreceptor switching is mainly due to variations in the V3 loop, while the mechanism needs to be further elucidated. We systematically studied the determinant for HIV-1 coreceptor switching by substitution of the genes from one R5 and one X4 pseudoviruses. The study results in successfully constructing two panels of chimeric viruses of R5 to X4 forward and X4 to R5 reverse switching. The determinants for tropism switching are the combined substitution of the V3 loop and C4 region of the HIV-1 envelope. The possible mechanism of the tropism switching includes two components, the V3 loop to enable the viral envelope binding to the newly switched coreceptor and the C4 region, to compensate for the loss of fitness caused by deleterious V3 loop mutations to maintain the overall viral viability. The combined C4 and V3 substitution showed at least an eightfold increase in replication activity compared with the pseudovirus with only V3 loop substitution. The site-directed mutations of N425R and S440-I442 with charged amino acids could especially increase viral activity. This study could facilitate HIV-1 phenotype surveillance and select right entry inhibitor, CCR5 or CXCR4 antagonists, for antiviral therapy.
Keywords: C4 region; HIV-1; V3 loop; coreceptor-switching; tropism.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Distinct molecular pathways to X4 tropism for a V3-truncated human immunodeficiency virus type 1 lead to differential coreceptor interactions and sensitivity to a CXCR4 antagonist.J Virol. 2010 Sep;84(17):8777-89. doi: 10.1128/JVI.00333-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573813 Free PMC article.
-
Covariation of Amino Acid Substitutions in the HIV-1 Envelope Glycoprotein gp120 and the Antisense Protein ASP Associated with Coreceptor Usage.Viruses. 2025 Feb 26;17(3):323. doi: 10.3390/v17030323. Viruses. 2025. PMID: 40143251 Free PMC article.
-
Role of naturally occurring basic amino acid substitutions in the human immunodeficiency virus type 1 subtype E envelope V3 loop on viral coreceptor usage and cell tropism.J Virol. 1999 Jul;73(7):5520-6. doi: 10.1128/JVI.73.7.5520-5526.1999. J Virol. 1999. PMID: 10364300 Free PMC article.
-
HIV-1 subtype C predicted co-receptor tropism in Africa: an individual sequence level meta-analysis.AIDS Res Ther. 2020 Feb 7;17(1):5. doi: 10.1186/s12981-020-0263-x. AIDS Res Ther. 2020. PMID: 32033571 Free PMC article. Review.
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
Cited by
-
Pseudotyped Viruses: A Useful Platform for Pre-Clinical Studies Conducted in a BSL-2 Laboratory Setting.Biomolecules. 2025 Jan 15;15(1):135. doi: 10.3390/biom15010135. Biomolecules. 2025. PMID: 39858529 Free PMC article. Review.
-
Multiple Mechanisms of HIV-1 Resistance to PGT135 in a Chinese Subtype B' Slow Progressor.Pathogens. 2025 Jun 3;14(6):556. doi: 10.3390/pathogens14060556. Pathogens. 2025. PMID: 40559564 Free PMC article.
-
Identification of a novel first-generation HIV-1 circulating recombinant form (CRF152_DG) among people living with HIV in Karachi, Pakistan.Microbiol Spectr. 2024 Jul 2;12(7):e0052924. doi: 10.1128/spectrum.00529-24. Epub 2024 May 21. Microbiol Spectr. 2024. PMID: 38771033 Free PMC article.
-
Distinct genetic clusters in HIV-1 CRF01_AE-infected patients induced variable degrees of CD4+ T-cell loss.mBio. 2024 Mar 13;15(3):e0334923. doi: 10.1128/mbio.03349-23. Epub 2024 Feb 22. mBio. 2024. PMID: 38385695 Free PMC article.
References
-
- Orloff GM, Orloff SL, Kennedy MS, et al. . Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J Immunol (Baltimore, MD: 1950). 1991;146(8):2578–2587. Epub 1991/04/15. PubMed PMID: 1673142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
