SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination
- PMID: 36647776
- PMCID: PMC10107710
- DOI: 10.1111/apm.13294
SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination
Abstract
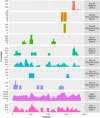
In Denmark, vaccination against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) has been with the Pfizer-BioNTech (BTN162b2) or the Moderna (mRNA-1273) mRNA vaccines. Patients with chronic hepatitis C virus (HCV) infection followed in our clinic received mRNA vaccinations according to the Danish roll-out vaccination plan. To monitor HCV infection, RNA was extracted from patient plasma and RNA sequencing was performed on the Illumina platform. In 10 of 108 HCV patient samples, full-length or traces of SARS-CoV-2 spike mRNA vaccine sequences were found in blood up to 28 days after COVID-19 vaccination. Detection of mRNA vaccine sequences in blood after vaccination adds important knowledge regarding this technology and should lead to further research into the design of lipid-nanoparticles and the half-life of these and mRNA vaccines in humans.
Keywords: Hepatitis C virus; SARS-CoV-2; blood; mRNA; vaccine.
© 2023 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Pathology, Medical Microbiology and Immunology.
Conflict of interest statement
Authors have no conflicts of interest to declare.
Figures
References
-
- Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, et al. Visualization of early events in mRNA vaccine delivery in non‐human primates via PET‐CT and near‐infrared imaging. Nat Biomed Eng. 2019;3:371–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

