Impact of epigenetic reprogramming on antitumor immune responses in glioma
- PMID: 36647827
- PMCID: PMC9843056
- DOI: 10.1172/JCI163450
Impact of epigenetic reprogramming on antitumor immune responses in glioma
Abstract
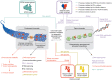
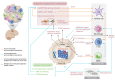
Epigenetic remodeling is a molecular hallmark of gliomas, and it has been identified as a key mediator of glioma progression. Epigenetic dysregulation contributes to gliomagenesis, tumor progression, and responses to immunotherapies, as well as determining clinical features. This epigenetic remodeling includes changes in histone modifications, chromatin structure, and DNA methylation, all of which are driven by mutations in genes such as histone 3 genes (H3C1 and H3F3A), isocitrate dehydrogenase 1/2 (IDH1/2), α-thalassemia/mental retardation, X-linked (ATRX), and additional chromatin remodelers. Although much of the initial research primarily identified how the epigenetic aberrations impacted glioma progression by solely examining the glioma cells, recent studies have aimed at establishing the role of epigenetic alterations in shaping the tumor microenvironment (TME). In this review, we discuss the mechanisms by which these epigenetic phenomena in glioma remodel the TME and how current therapies targeting epigenetic dysregulation affect the glioma immune response and therapeutic outcomes. Understanding the link between epigenetic remodeling and the glioma TME provides insights into the implementation of epigenetic-targeting therapies to improve the antitumor immune response.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

