Dysphagia Days as an Assessment of Clinical Treatment Outcome in Eosinophilic Esophagitis
- PMID: 36647838
- PMCID: PMC10045973
- DOI: 10.14309/ajg.0000000000002094
Dysphagia Days as an Assessment of Clinical Treatment Outcome in Eosinophilic Esophagitis
Abstract
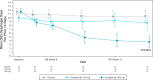
Introduction: The aim of this study was to evaluate Dysphagia Days as a measure of symptom improvement in patients with eosinophilic esophagitis from the HEROES study.
Methods: Dysphagia Days, defined as a yes answer to the following question: During any meal today, did food go down slowly or get stuck in your throat or chest? was assessed for cendakimab vs placebo.
Results: A statistically significant reduction in the mean number of Dysphagia Days experienced was observed with cendakimab 360 mg vs placebo at week 16 (-4.67 vs -1.83; P = 0.0115); an even greater improvement was observed in steroid-refractory patients vs placebo (-4.48 vs -0.04; P = 0.0079).
Discussion: Dysphagia Days represents a relevant clinical end point to capture dysphagia-related symptoms.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Dellon ES, Irani AM, Hill MR, et al. . Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther 2013;38(6):634–42. - PubMed
-
- Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol 2007;120(6):1292–300. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

