Impact of aging on mitochondrial respiration in various organs
- PMID: 36647911
- PMCID: PMC9906668
- DOI: 10.33549/physiolres.934995
Impact of aging on mitochondrial respiration in various organs
Abstract
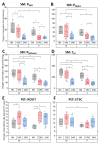
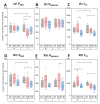
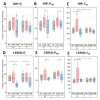
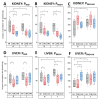
Mitochondria are considered central regulator of the aging process; however, majority of studies dealing with the impact of age on mitochondrial oxygen consumption focused on skeletal muscle concluding (although not uniformly) a general declining trend with advancing age. In addition, gender related differences in mitochondrial respiration have not been satisfactorily described yet. The aim of the present study was to evaluate mitochondrial oxygen consumption in various organs of aging male and female Fischer 344 rats at the ages of 6, 12 and 24 months. Mitochondrial respiration of homogenized (skeletal muscle, left and right heart ventricle, hippocampus, cerebellum, kidney cortex), gently mechanically permeabilized (liver) tissue or intact cells (platelets) was determined using high-resolution respirometry (oxygraphs O2k, Oroboros, Austria). The pattern of age-related changes differed in each tissue: in the skeletal muscle and kidney cortex of both sexes and in female heart, parameters of mitochondrial respiration significantly declined with age. Resting respiration of intact platelets displayed an increasing trend and it did not correlate with skeletal muscle respiratory states. In the heart of male rats and brain tissues of both sexes, respiratory states remained relatively stable over analyzed age categories with few exceptions of lower mitochondrial oxygen consumption at the age of 24 months. In the liver, OXPHOS capacity was higher in females than in males with either no difference between the ages of 6 and 24 months or even significant increase at the age of 24 months in the male rats. In conclusion, the results of our study indicate that the concept of general pattern of age-dependent decline in mitochondrial oxygen consumption across different organs and tissues could be misleading. Also, the statement of higher mitochondrial respiration in females seems to be conflicting, since the gender-related differences may vary with the tissue studied, combination of substrates used and might be better detectable at younger ages than in old animals.
Conflict of interest statement
There is no conflict of interest.
Figures
Similar articles
-
Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging.Aging Cell. 2018 Apr;17(2):e12725. doi: 10.1111/acel.12725. Epub 2018 Jan 21. Aging Cell. 2018. PMID: 29356348 Free PMC article.
-
A comparative study of mitochondrial respiration in circulating blood cells and skeletal muscle fibers in women.Am J Physiol Endocrinol Metab. 2019 Sep 1;317(3):E503-E512. doi: 10.1152/ajpendo.00084.2019. Epub 2019 Jun 18. Am J Physiol Endocrinol Metab. 2019. PMID: 31211617
-
Normal to enhanced intrinsic mitochondrial respiration in skeletal muscle of middle- to older-aged women and men with uncomplicated type 1 diabetes.Diabetologia. 2021 Nov;64(11):2517-2533. doi: 10.1007/s00125-021-05540-1. Epub 2021 Aug 14. Diabetologia. 2021. PMID: 34392397
-
Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology.Int J Biochem Cell Biol. 2009 Oct;41(10):1837-45. doi: 10.1016/j.biocel.2009.03.013. Epub 2009 Apr 2. Int J Biochem Cell Biol. 2009. PMID: 19467914 Review.
-
Impact of aging on mitochondrial function in cardiac and skeletal muscle.Free Radic Biol Med. 2016 Sep;98:177-186. doi: 10.1016/j.freeradbiomed.2016.03.017. Epub 2016 Mar 24. Free Radic Biol Med. 2016. PMID: 27033952 Review.
Cited by
-
Moderate-intensity interval exercise exacerbates cardiac lipotoxicity in high-fat, high-calories diet-fed mice.Nat Commun. 2025 Jan 12;16(1):613. doi: 10.1038/s41467-025-55917-8. Nat Commun. 2025. PMID: 39800728 Free PMC article.
-
Identification of aging-related biomarkers for intervertebral disc degeneration in whole blood samples based on bioinformatics and machine learning.Front Immunol. 2025 Apr 15;16:1565945. doi: 10.3389/fimmu.2025.1565945. eCollection 2025. Front Immunol. 2025. PMID: 40303407 Free PMC article.
-
Mitochondrial function in peripheral blood cells across the human lifespan.NPJ Aging. 2024 Feb 7;10(1):10. doi: 10.1038/s41514-023-00130-4. NPJ Aging. 2024. PMID: 38326348 Free PMC article.
-
Young adult and aged female rats are vulnerable to amygdala-dependent, but not hippocampus-dependent, memory impairment following short-term high-fat diet.Brain Res Bull. 2023 Apr;195:145-156. doi: 10.1016/j.brainresbull.2023.03.001. Epub 2023 Mar 2. Brain Res Bull. 2023. PMID: 36870621 Free PMC article. Review.
-
A robust method for assessing mitochondrial function in healthy and diseased frozen cardiac tissue.Commun Biol. 2025 Aug 19;8(1):1249. doi: 10.1038/s42003-025-08608-5. Commun Biol. 2025. PMID: 40830221 Free PMC article.
