Sex and prior exposure jointly shape innate immune responses to a live herpesvirus vaccine
- PMID: 36648132
- PMCID: PMC9844983
- DOI: 10.7554/eLife.80652
Sex and prior exposure jointly shape innate immune responses to a live herpesvirus vaccine
Abstract
Background: Both sex and prior exposure to pathogens are known to influence responses to immune challenges, but their combined effects are not well established in humans, particularly in early innate responses critical for shaping subsequent outcomes.
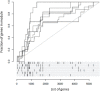
Methods: We employed systems immunology approaches to study responses to a replication-defective, herpes simplex virus (HSV) 2 vaccine in men and women either naive or previously exposed to HSV.
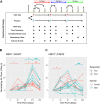
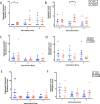
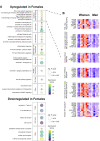
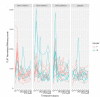
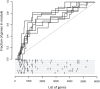
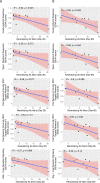
Results: Blood transcriptomic and cell population profiling showed substantial changes on day 1 after vaccination, but the responses depended on sex and whether the vaccinee was naive or previously exposed to HSV. The magnitude of early transcriptional responses was greatest in HSV naive women where type I interferon (IFN) signatures were prominent and associated negatively with vaccine-induced neutralizing antibody titers, suggesting that a strong early antiviral response reduced the uptake of this replication-defective virus vaccine. While HSV seronegative vaccine recipients had upregulation of gene sets in type I IFN (IFN-α/β) responses, HSV2 seropositive vaccine recipients tended to have responses focused more on type II IFN (IFN-γ) genes.
Conclusions: These results together show that prior exposure and sex interact to shape early innate responses that then impact subsequent adaptive immune phenotypes.
Funding: Intramural Research Program of the NIH, the National Institute of Allergy and Infectious Diseases, and other institutes supporting the Trans-NIH Center for Human Immunology, Autoimmunity, and Inflammation. The vaccine trial was supported through a clinical trial agreement between the National Institute of Allergy and Infectious Diseases and Sanofi Pasteur. Clinical trial number: NCT01915212.
Keywords: herpes simplex; human; infectious disease; innate immunity; medicine; microbiology; sex dimorphism; systems immunology; transcriptomics; vaccine.
Conflict of interest statement
FC, RA, LD, YK, JC, TJ, ML, JC, AB, KH, NR, HP, KW, JT, JC No competing interests declared
Figures



References
-
- Aaby P, Martins CL, Garly M-L, Andersen A, Fisker AB, Claesson MH, Ravn H, Rodrigues A, Whittle HC, Benn CS. Measles vaccination in the presence or absence of maternal measles antibody: impact on child survival. Clinical Infectious Diseases. 2014;59:484–492. doi: 10.1093/cid/ciu354. - DOI - PMC - PubMed
-
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women Efficacy results of a trial of a herpes simplex vaccine. The New England Journal of Medicine. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. - DOI - PMC - PubMed
-
- Bernstein DI, Aoki FY, Tyring SK, Stanberry LR, St-Pierre C, Shafran SD, Leroux-Roels G, Van Herck K, Bollaerts A, Dubin G, GlaxoSmithKline Herpes Vaccine Study Group Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clinical Infectious Diseases. 2005;40:1271–1281. doi: 10.1086/429240. - DOI - PubMed
-
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Niño D, Arden N, Quarles JM, Couch RB, Belmont JW. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. The Journal of Infectious Diseases. 2011;203:921–929. doi: 10.1093/infdis/jiq156. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

