A Stable Dried Tube Specimen for Quality Assurance and Training Programs for HIV Rapid Test for Recent Infection
- PMID: 36648237
- PMCID: PMC9927143
- DOI: 10.1128/spectrum.03398-22
A Stable Dried Tube Specimen for Quality Assurance and Training Programs for HIV Rapid Test for Recent Infection
Abstract
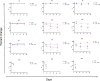
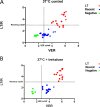
The HIV epidemic is still one of the world's most serious public health challenges, affecting about 38 million people worldwide, especially in sub-Saharan African and Southeast Asian countries. In recent years, tests have been developed to discriminate recent from long-term infection in HIV-infected populations, and these tools can help identify new outbreaks and networks of transmission and target prevention and treatment plans. New rapid tests for recent infection are being deployed in point-of-care settings; however, quality assurance programs need to be implemented to ensure consistency and reliability of the results. We have developed a dried tube specimen (DTS) stabilized with disaccharide trehalose as a quality control reagent for rapid recency testing that can be stored unrefrigerated prior to reconstitution at temperatures up to 37°C for up to 12 weeks. Analysis of 10 trehalose-stabilized DTSs showed that they maintained the same recency classification in all of the samples stored at 4°C and 37°C up to 12 weeks and at 56°C for 2 weeks, while the DTSs prepared without trehalose changed their classification from long-term to recent or recent to negative after storage at 37°C for 12 weeks. Development of DTS quality control reagents will facilitate proficiency and training programs, particularly in settings without cold chain capability in field environments. IMPORTANCE Implementation of stabilized dried tube specimens (DTSs) for quality control and training would facilitate HIV recency programs, especially in point-of-care settings without cold chain availability. This study shows that addition of the disaccharide trehalose to DTSs prior to drying the samples increased stability of the samples across a range of temperatures. This finding provides an affordable way to increase the availability of these key reagents for quality control in resource-constrained settings.
Keywords: HIV rapid test; HIV recency infection; human immunodeficiency virus.
Conflict of interest statement
The authors declare a conflict of interest. As an inventor of rapid test for recent infection, B.S.P. receives a portion of royalties from the sale of Asante Rapid Recency Assay as per policy of the U.S. Government. The assay is manufactured by Sedia BioSciences under a technology transfer licensing agreement with C.D.C. This does not compromise author's obligation to ensure accuracy and integrity of the data presented in this manuscript. No other authors have conflicts of interests to declare.
Figures


Similar articles
-
Dried tube specimens: a simple and cost-effective method for preparation of HIV proficiency testing panels and quality control materials for use in resource-limited settings.J Virol Methods. 2010 Feb;163(2):295-300. doi: 10.1016/j.jviromet.2009.10.013. Epub 2009 Oct 28. J Virol Methods. 2010. PMID: 19878697
-
Generation of dried tube specimen for HIV-1 viral load proficiency test panels: a cost-effective alternative for external quality assessment programs.J Virol Methods. 2013 Mar;188(1-2):1-5. doi: 10.1016/j.jviromet.2012.11.036. Epub 2012 Dec 7. J Virol Methods. 2013. PMID: 23219930
-
Monitoring the quality of HIV-1 viral load testing through a proficiency testing program using dried tube specimens in resource-limited settings.J Clin Microbiol. 2015 Apr;53(4):1129-36. doi: 10.1128/JCM.02780-14. Epub 2015 Jan 21. J Clin Microbiol. 2015. PMID: 25609733 Free PMC article.
-
HIV-1 viral load testing in resource-limited settings: Challenges and solutions for specimen integrity.Rev Med Virol. 2021 Mar;31(2):e2165. doi: 10.1002/rmv.2165. Epub 2020 Sep 25. Rev Med Virol. 2021. PMID: 32978882 Review.
-
AIDS in sub-Saharan Africa: the epidemiology of heterosexual transmission and the prospects for prevention.Epidemiology. 1993 Jan;4(1):63-72. Epidemiology. 1993. PMID: 8420583 Review.
Cited by
-
Innovating Quality Control and External Quality Assurance for HIV-1 Recent Infection Testing: Empowering HIV Surveillance in Lao PDR.Viruses. 2025 Jul 17;17(7):1004. doi: 10.3390/v17071004. Viruses. 2025. PMID: 40733620 Free PMC article.
References
-
- UNAIDS. 2019. AIDSinfo. UNAIDS, Geneva, Switzerland. https://aidsinfo.unaids.org/.

