Recurrent venous thromboembolism and bleeding with extended anticoagulation: the VTE-PREDICT risk score
- PMID: 36648242
- PMCID: PMC10079391
- DOI: 10.1093/eurheartj/ehac776
Recurrent venous thromboembolism and bleeding with extended anticoagulation: the VTE-PREDICT risk score
Erratum in
-
Correction to: Recurrent venous thromboembolism and bleeding with extended anticoagulation: the VTE-PREDICT risk score.Eur Heart J. 2024 Aug 3;45(29):2630. doi: 10.1093/eurheartj/ehae396. Eur Heart J. 2024. PMID: 38917060 Free PMC article. No abstract available.
Abstract
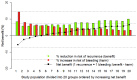
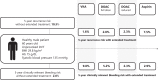
Aims: Deciding to stop or continue anticoagulation for venous thromboembolism (VTE) after initial treatment is challenging, as individual risks of recurrence and bleeding are heterogeneous. The present study aimed to develop and externally validate models for predicting 5-year risks of recurrence and bleeding in patients with VTE without cancer who completed at least 3 months of initial treatment, which can be used to estimate individual absolute benefits and harms of extended anticoagulation.
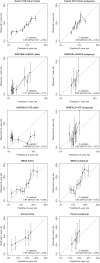
Methods and results: Competing risk-adjusted models were derived to predict recurrent VTE and clinically relevant bleeding (non-major and major) using 14 readily available patient characteristics. The models were derived from combined individual patient data from the Bleeding Risk Study, Hokusai-VTE, PREFER-VTE, RE-MEDY, and RE-SONATE (n = 15,141, 220 recurrences, 189 bleeding events). External validity was assessed in the Danish VTE cohort, EINSTEIN-CHOICE, GARFIELD-VTE, MEGA, and Tromsø studies (n = 59 257, 2283 recurrences, 3335 bleeding events). Absolute treatment effects were estimated by combining the models with hazard ratios from trials and meta-analyses. External validation in different settings showed agreement between predicted and observed risks up to 5 years, with C-statistics ranging from 0.48-0.71 (recurrence) and 0.61-0.68 (bleeding). In the Danish VTE cohort, 5-year risks ranged from 4% to 19% for recurrent VTE and 1% -19% for bleeding.
Conclusion: The VTE-PREDICT risk score can be applied to estimate the effect of extended anticoagulant treatment for individual patients with VTE and to support shared decision-making.
Keywords: Anticoagulants; Haemorrhage; Prediction model; Recurrence; Risk; Venous thromboembolism.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of Interest: Marc Carrier reports research funding from BMS, Leo Pharma, and Pfizer, and honoraria from Bayer, Sanofi, BMS, Leo Pharma, Servier and Pfizer. All fees are paid to his institution. Ander Cohen reports grants or contracts, consulting fees and payment or honoraria from Bayer AG, Daiichi Sankyo, BMS/Pfizer, and AstraZeneca. All fees were paid to his company. Alfredo Farjat was a full-time employee at Thrombosis Research Institute when he collaborated with this study. Akos Ference Pap is an employee of Bayer AG. Samuel Goldhaber reports grants or contracts from Bayer, Boston Scientific BTG EKOS, NHLBI, BMS, Janssen and Pfizer, consulting fees from Agile, Pfizer, Bayer, speaker fees from Lankenau Grand Rounds in Medicine, Bakken Symposium -University of Minnesota, The Brigham Board Review in Critical Care ‘Virtual Studio/Distance Learning’, New York Cardiovascular Symposium, Latin American Anticoagulation Series Conference, Jeresaty Symposium—Trinity Health of New England, Rutgers New Jersey Medical School Grand Rounds, SBACV Symposium—Brazil Society of Angiology and Vascular Medicine, Brigham-Sheba Collaboration on Thrombosis and Vascular Medicine and Philippine Society of Vascular Medicine Annual Convention. Michael Grosso is an employee of Daiichi Sankyo. Ajay Kakkar reports grants from Bayer AG, Sanofi SA, personal fees from Anthos Therapeutics, Bayer AG, Sanofi S.A. Karen Pieper reports consulting fees from Cryolife, J&J Pharmaceuticals, Artivion and Element Science. Gary Raskob reports consultancy fees or honoraria from AMAG Pharma, Anylam, Anthos Therapeutics, Bayer HealthCare Pharmaceuticals Inc., Bristol-Myers Squibb, Daiichi Sankyo Inc., Janssen Global Services LLC, Pfizer, and XaTrek; honoraria from BMS, Pfizer, Daiichi Sankyo; DSMB or advisory board membership from Anthos Therapetuics, Janssen, Bristol-Myers Squibb and Pfizer, leadership or fiduciary role in other board, society, committee or advocacy group of OU Health, stock or stock option ownership for AbbVie, Inc., Gilead Sciences Inc, GlaxoSmithKline, LLC., LLY, MRK, and Pfizer. Sam Schulman reports research grants, paid to his institution, from Octopharma, and consulting fees from Alexion, Bayer, Boehringer–Ingelheim, Sanofi, Daiichi Sankyo and Octopharma. Minggao Shi is an employee of Daiichi Sankyo. Phillip Wells reports grants and speaker fees from BMS/Pfizer, consulting fees from Anthos and speaker fees and consulting fees from Bayer Healthcare. All fees are paid to his institution. The other authors have nothing to declare.
Figures


Comment in
-
Walking the tightrope: a balanced discussion of the benefits and harms of extended duration anticoagulation for venous thrombo-embolism.Eur Heart J. 2023 Apr 7;44(14):1245-1247. doi: 10.1093/eurheartj/ehac731. Eur Heart J. 2023. PMID: 36656796 No abstract available.
-
Focus issue on vascular biology and medicine spanning from management of stroke to new therapeutic targets in aortic dissection and pulmonary hypertension.Eur Heart J. 2023 Apr 7;44(14):1193-1196. doi: 10.1093/eurheartj/ehad198. Eur Heart J. 2023. PMID: 37024112 No abstract available.
-
In adults with VTE who received anticoagulants for ≥3 mo, VTE-PREDICT predicted recurrence and bleeding at up to 5 y.Ann Intern Med. 2023 May;176(5):JC59. doi: 10.7326/J23-0021. Epub 2023 May 2. Ann Intern Med. 2023. PMID: 37126813
References
-
- Konstantinides S V, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2019;41:543–603. 10.1093/eurheartj/ehz405 - DOI - PubMed
-
- Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. . Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036. 10.1136/bmj.d3036 - DOI - PMC - PubMed

