FGF21 protects against hepatic lipotoxicity and macrophage activation to attenuate fibrogenesis in nonalcoholic steatohepatitis
- PMID: 36648330
- PMCID: PMC9928421
- DOI: 10.7554/eLife.83075
FGF21 protects against hepatic lipotoxicity and macrophage activation to attenuate fibrogenesis in nonalcoholic steatohepatitis
Abstract
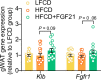
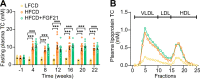
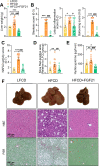
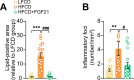
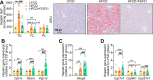
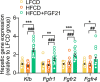
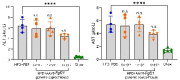
Analogues of the hepatokine fibroblast growth factor 21 (FGF21) are in clinical development for type 2 diabetes and nonalcoholic steatohepatitis (NASH) treatment. Although their glucose-lowering and insulin-sensitizing effects have been largely unraveled, the mechanisms by which they alleviate liver injury have only been scarcely addressed. Here, we aimed to unveil the mechanisms underlying the protective effects of FGF21 on NASH using APOE*3-Leiden.CETP mice, a well-established model for human-like metabolic diseases. Liver-specific FGF21 overexpression was achieved in mice, followed by administration of a high-fat high-cholesterol diet for 23 weeks. FGF21 prevented hepatic lipotoxicity, accompanied by activation of thermogenic tissues and attenuation of adipose tissue inflammation, improvement of hyperglycemia and hypertriglyceridemia, and upregulation of hepatic programs involved in fatty acid oxidation and cholesterol removal. Furthermore, FGF21 inhibited hepatic inflammation, as evidenced by reduced Kupffer cell (KC) activation, diminished monocyte infiltration, and lowered accumulation of monocyte-derived macrophages. Moreover, FGF21 decreased lipid- and scar-associated macrophages, which correlated with less hepatic fibrosis as demonstrated by reduced collagen accumulation. Collectively, hepatic FGF21 overexpression limits hepatic lipotoxicity, inflammation, and fibrogenesis. Mechanistically, FGF21 blocks hepatic lipid influx and accumulation through combined endocrine and autocrine signaling, respectively, which prevents KC activation and lowers the presence of lipid- and scar-associated macrophages to inhibit fibrogenesis.
Keywords: fibroblast growth factor 21; immunology; inflammation; lipid/scar-associated macrophages; liver-adipose tissue crosstalk; medicine; mouse; steatohepatitis.
Plain language summary
High-calorie modern diets have contributed to growing rates of obesity-linked diseases. One such disease is non-alcoholic steatohepatitis or NASH for short, which affects about 5% of adults in the United States. The livers of people with this condition accumulate fat, become inflamed, and develop scar tissue. People with NASH are also at increased risk of developing liver cancer, type 2 diabetes, and heart disease. Currently, no drugs are available to treat the condition and prevent such severe complications. Previous research has shown the liver produces a stress hormone, called FGF21, in response to fat accumulation. This hormone boosts fat burning and so helps to reduce excess fat in the liver. Drugs that mimic FGF21 have already been developed for type 2 diabetes. But so far, it was unclear if such drugs could also help reduce liver inflammation and scarring in patients with NASH. Liu et al. show that increasing the production of FGF21 in mice with a NASH-like condition reduces fat accumulation, liver inflammation, and scarring. In the experiments, the researchers used gene therapy to ramp up FGF21 production in the livers of mice that develop obesity and a NASH-like condition when fed a high-fat diet for 23 weeks. Increasing FGF21 production prevented the mice from developing obesity while on the high fat diet by making the body burn more fat in the liver and brown fat tissue. The treatment also reduced inflammation and prevented scarring by reducing the number and activity of immune cells in the liver. Increasing the production of the stress hormone FGF21 prevents diet-induced obesity and NASH in mice fed a high-fat diet. More studies are necessary to determine if using gene therapy to increase FGF21 may also cause weight loss and could reverse liver damage in mice that already have NASH. If this approach is effective in mice, it may be tested in humans, a process that may take several years. If human studies are successful, FGF21-boosting therapy might provide a new treatment approach for obesity or NASH.
© 2023, Liu et al.
Conflict of interest statement
CL, MS, BS, JL, Hv, EZ, MT, BG, MB, YW, PR No competing interests declared, AA, AP, SO, MU, IA, YI, KW, XP employee of AstraZeneca
Figures








References
-
- Aggarwal P, Noureddin M, Harrison S, Jeannin S, Alkhouri N. Nonalcoholic steatohepatitis (NASH) cirrhosis: a snapshot of therapeutic agents in clinical development and the optimal design for clinical trials. Expert Opinion on Investigational Drugs. 2022;31:163–172. doi: 10.1080/13543784.2022.2032640. - DOI - PubMed
-
- Bao LC, Yin J, Gao W, Wang Q, Yao WB, Gao XD. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. British Journal of Pharmacology. 2018;175:3379–3393. doi: 10.1111/bph.14383. - DOI - PMC - PubMed
-
- Barb D, Bril F, Kalavalapalli S, Cusi K. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2019;104:3327–3336. doi: 10.1210/jc.2018-02414. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

