Progress in fed-batch culture for recombinant protein production in CHO cells
- PMID: 36648523
- PMCID: PMC9843118
- DOI: 10.1007/s00253-022-12342-x
Progress in fed-batch culture for recombinant protein production in CHO cells
Abstract
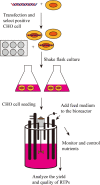
Nearly 80% of the approved human therapeutic antibodies are produced by Chinese Hamster Ovary (CHO) cells. To achieve better cell growth and high-yield recombinant protein, fed-batch culture is typically used for recombinant protein production in CHO cells. According to the demand of nutrients consumption, feed medium containing multiple components in cell culture can affect the characteristics of cell growth and improve the yield and quality of recombinant protein. Fed-batch optimization should have a connection with comprehensive factors such as culture environmental parameters, feed composition, and feeding strategy. At present, process intensification (PI) is explored to maintain production flexible and meet forthcoming demands of biotherapeutics process. Here, CHO cell culture, feed composition in fed-batch culture, fed-batch culture environmental parameters, feeding strategies, metabolic byproducts in fed-batch culture, chemostat cultivation, and the intensified fed-batch are reviewed. KEY POINTS: • Fed-batch culture in CHO cells is reviewed. • Fed-batch has become a common technology for recombinant protein production. • Fed batch culture promotes recombinant protein production in CHO cells.
Keywords: CHO cell; Fed-batch culture; Process development; Recombinant therapeutic proteins.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Baik JY, Dahodwala H, Oduah E, Talman L, Gemmill TR, Gasimli L, Datta P, Yang B, Li G, Zhang F, Li L, Linhardt RJ, Campbell AM, Gorfien SF, Sharfstein ST. Optimization of bioprocess conditions improves production of a CHO cell-derived, bioengineered heparin. Biotechnol J. 2015;10:1067–1081. doi: 10.1002/biot.201400665. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

