Association of Body Weight With Response to Vitamin D Supplementation and Metabolism
- PMID: 36648947
- PMCID: PMC9856931
- DOI: 10.1001/jamanetworkopen.2022.50681
Association of Body Weight With Response to Vitamin D Supplementation and Metabolism
Abstract
Importance: In the Vitamin D and Omega-3 Trial (VITAL), the effects of randomized vitamin D supplementation (cholecalciferol), 2000 IU/d, reduced the risk of several health outcomes among participants with normal, but not elevated, body weights. It was unclear whether weight had any association with the outcomes of the supplementation.
Objective: To investigate whether baseline body mass index (BMI) modifies vitamin D metabolism and response to supplementation.
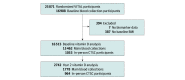
Design, setting, and participants: VITAL is a completed randomized, double-blind, placebo-controlled trial for the primary prevention of cancer and cardiovascular disease. In the present cohort study, an analysis was conducted in a subset of VITAL participants who provided a blood sample at baseline and a subset with a repeated sample at 2 years' follow-up. VITAL was conducted from July 1, 2010, to November 10, 2018; data analysis for the present study was conducted from August 1, 2021, to November 9, 2021.
Interventions: Treatment outcomes of vitamin D, 2000 IU/d, supplementation vs placebo associated with clinical and novel vitamin D-related biomarkers by BMI category adjusted for other factors associated with vitamin D status.
Main outcomes and measures: Multivariable-adjusted means (SE) or 95% CIs of vitamin D-related serum biomarkers at baseline and follow-up: total 25-hydroxyvitamin D (25-OHD), 25-OHD3, free vitamin D (FVD), bioavailable vitamin D (BioD), vitamin D-binding protein (VDBP), albumin, parathyroid hormone (PTH), and calcium, and log-transformed as needed.
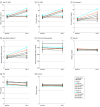
Results: A total of 16 515 participants (mean [SD] age, 67.7 [7.0] years; 8371 women [50.7%]; 12420 non-Hispanic White [76.9%]) were analyzed at baseline, including 2742 with a follow-up blood sample. Before randomization, serum total 25-OHD levels were incrementally lower at higher BMI categories (adjusted mean [SE]: underweight, 32.3 [0.7] ng/mL; normal weight, 32.3 [0.1] ng/mL; overweight, 30.5 [0.1] ng/mL; obesity class I, 29.0 [0.2] ng/mL; and obesity class II, 28.0 [0.2] ng/mL; P < .001 for linear trend). Similarly, baseline 25-OHD3, FVD, BioD, VDBP, albumin, and calcium levels were lower with higher BMI, while PTH level was higher (all P < .001 for linear trend). Compared with placebo, randomization to vitamin D supplementation was associated with an increase in total 25-OHD, 25-OHD3, FVD, and BioD levels compared with placebo at 2 years' follow-up, but increases were significantly lower at higher BMI categories (all treatment effect interactions P < .001). Supplementation did not substantially change VDBP, albumin, PTH, or calcium levels.
Conclusions and relevance: In this randomized cohort study, vitamin D supplementation increased serum vitamin D-related biomarkers, with a blunted response observed for participants with overweight or obesity at baseline. These longitudinal findings suggest that BMI may be associated with modified response to vitamin D supplementation and may in part explain the observed diminished outcomes of supplementation for various health outcomes among individuals with higher BMI.
Conflict of interest statement
Figures
Comment in
-
Responses to Vitamin D Supplementation in Individuals With Overweight and Obesity.JAMA Netw Open. 2023 Jan 3;6(1):e2250695. doi: 10.1001/jamanetworkopen.2022.50695. JAMA Netw Open. 2023. PMID: 36648948 No abstract available.

