Analytical Validation of SOD2 Genotyping
- PMID: 36648953
- PMCID: PMC9844328
- DOI: 10.3390/mps6010004
Analytical Validation of SOD2 Genotyping
Abstract
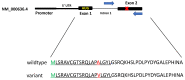
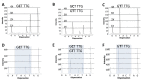
Manganese superoxide dismutase-2 (SOD2) plays a crucial role in cells' protection against mitochondrial oxidative damage. A genetic polymorphism in the mitochondrial targeting sequence of the SOD2 gene has been implicated in various diseases, including prostate cancer. Paller et al. have shown an increase in prostate-specific antigen (PSA) doubling time in patients with the Ala/Ala (wildtype) genotype when treated with pomegranate/grape extract antioxidants. We developed and validated a pyrosequencing assay that detects the common germline SOD2 SNP (rs_4880) with the aim of identifying men with castrate-resistant prostate cancer eligible for an antioxidant therapy clinical trial. We first selected 37 samples from the 1000 genomes study with known genotypes determined using Illumina-based sequencing and confirmed them by Sanger sequencing. In a blinded design, we then performed the new pyrosequencing assay on these samples and assigned genotypes. Genotypes for all 37 samples (13 homozygous Ala, 12 heterozygous Ala/Val, and 12 homozygous Val) were all concordant by pyrosequencing. The pyrosequencing assay has been live since May 2018 and has proven to be robust and accurate.
Keywords: SOD2 gene; prostate cancer; prostate-specific antigen (PSA); pyrosequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McDermott C.L., Blough D.K., Fedorenko C.R., Arora N.K., Zeliadt S.B., Fairweather M.E., Oakley-Girvan I., Van Den Eeden S.K., Ramsey S.D. Complementary and alternative medicine use among newly diagnosed prostate cancer patients. Support. Care Cancer. 2012;20:65–73. doi: 10.1007/s00520-010-1055-y. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

