TFEB-mediated lysosomal exocytosis alleviates high-fat diet-induced lipotoxicity in the kidney
- PMID: 36649084
- PMCID: PMC9977505
- DOI: 10.1172/jci.insight.162498
TFEB-mediated lysosomal exocytosis alleviates high-fat diet-induced lipotoxicity in the kidney
Abstract
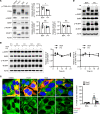
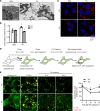
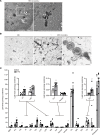
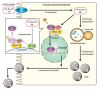
Obesity is a major risk factor for end-stage kidney disease. We previously found that lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in obesity-related kidney disease, in both humans and experimental animal models. However, the regulatory factors involved in countering renal lipotoxicity are largely unknown. Here, we found that palmitic acid strongly promoted dephosphorylation and nuclear translocation of transcription factor EB (TFEB) by inhibiting the mechanistic target of rapamycin kinase complex 1 pathway in a Rag GTPase-dependent manner, though these effects gradually diminished after extended treatment. We then investigated the role of TFEB in the pathogenesis of obesity-related kidney disease. Proximal tubular epithelial cell-specific (PTEC-specific) Tfeb-deficient mice fed a high-fat diet (HFD) exhibited greater phospholipid accumulation in enlarged lysosomes, which manifested as multilamellar bodies (MLBs). Activated TFEB mediated lysosomal exocytosis of phospholipids, which helped reduce MLB accumulation in PTECs. Furthermore, HFD-fed, PTEC-specific Tfeb-deficient mice showed autophagic stagnation and exacerbated injury upon renal ischemia/reperfusion. Finally, higher body mass index was associated with increased vacuolation and decreased nuclear TFEB in the proximal tubules of patients with chronic kidney disease. These results indicate a critical role of TFEB-mediated lysosomal exocytosis in counteracting renal lipotoxicity.
Keywords: Chronic kidney disease; Lysosomes; Metabolism; Nephrology; Obesity.
Figures





References
-
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

