Intranasal delivery of full-length anti-Nogo-A antibody: A potential alternative route for therapeutic antibodies to central nervous system targets
- PMID: 36649432
- PMCID: PMC9942809
- DOI: 10.1073/pnas.2200057120
Intranasal delivery of full-length anti-Nogo-A antibody: A potential alternative route for therapeutic antibodies to central nervous system targets
Abstract
Antibody delivery to the CNS remains a huge hurdle for the clinical application of antibodies targeting a CNS antigen. The blood-brain barrier and blood-CSF barrier restrict access of therapeutic antibodies to their CNS targets in a major way. The very high amounts of therapeutic antibodies that are administered systemically in recent clinical trials to reach CNS targets are barely viable cost-wise for broad, routine applications. Though global CNS delivery of antibodies can be achieved by intrathecal application, these procedures are invasive. A non-invasive method to bring antibodies into the CNS reliably and reproducibly remains an important unmet need in neurology. In the present study, we show that intranasal application of a mouse monoclonal antibody against the neurite growth-inhibiting and plasticity-restricting membrane protein Nogo-A leads to a rapid transfer of significant amounts of antibody to the brain and spinal cord in intact adult rats. Daily intranasal application for 2 wk of anti-Nogo-A antibody enhanced growth and compensatory sprouting of corticofugal projections and functional recovery in rats after large unilateral cortical strokes. These findings are a starting point for clinical translation for a less invasive route of application of therapeutic antibodies to CNS targets for many neurological indications.
Keywords: Nogo-A; antibody therapy; intranasal; neurodegeneration; stroke recovery.
Conflict of interest statement
M.E.S. is the co-founder and president of the board of NovaGo Therapeutics AG. All other authors declare that they have no competing interests.
Figures

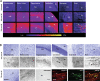


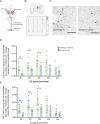

Similar articles
-
Targeting Therapeutic Antibodies to the CNS: a Comparative Study of Intrathecal, Intravenous, and Subcutaneous Anti-Nogo A Antibody Treatment after Stroke in Rats.Neurotherapeutics. 2020 Jul;17(3):1153-1159. doi: 10.1007/s13311-020-00864-z. Neurotherapeutics. 2020. PMID: 32378027 Free PMC article.
-
Intrathecally infused antibodies against Nogo-A penetrate the CNS and downregulate the endogenous neurite growth inhibitor Nogo-A.Mol Cell Neurosci. 2006 May-Jun;32(1-2):161-73. doi: 10.1016/j.mcn.2006.03.007. Epub 2006 May 11. Mol Cell Neurosci. 2006. PMID: 16697217
-
Intrathecal treatment with anti-Nogo-A antibody improves functional recovery in adult rats after stroke.Exp Brain Res. 2007 Sep;182(2):261-6. doi: 10.1007/s00221-007-1067-0. Epub 2007 Aug 24. Exp Brain Res. 2007. PMID: 17717658
-
Anti-Nogo on the go: from animal models to a clinical trial.Ann N Y Acad Sci. 2010 Jun;1198 Suppl 1:E22-34. doi: 10.1111/j.1749-6632.2010.05566.x. Ann N Y Acad Sci. 2010. PMID: 20590535 Review.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
Cited by
-
The PSD-95 inhibitor NA-1 is delivered to the brain upon nasal administration with uptake into the olfactory bulb improved by co-administration with the cell-penetrating peptides lowPro and Tat.Drug Deliv Transl Res. 2025 Apr 3. doi: 10.1007/s13346-025-01842-8. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40180762
-
Intranasal Treatment with Cannabinoid 2 Receptor Agonist HU-308 Ameliorates Cold Sensitivity in Mice with Traumatic Trigeminal Neuropathic Pain.Cells. 2024 Nov 22;13(23):1943. doi: 10.3390/cells13231943. Cells. 2024. PMID: 39682692 Free PMC article.
-
Clearance of erythrocytes from the subarachnoid space through cribriform plate lymphatics in female mice.EBioMedicine. 2024 Sep;107:105295. doi: 10.1016/j.ebiom.2024.105295. Epub 2024 Aug 22. EBioMedicine. 2024. PMID: 39178745 Free PMC article.
-
Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders.Pharmaceutics. 2023 Dec 31;16(1):66. doi: 10.3390/pharmaceutics16010066. Pharmaceutics. 2023. PMID: 38258077 Free PMC article. Review.
-
Recent advances in immunotherapy targeting amyloid-beta and tauopathies in Alzheimer's disease.Neural Regen Res. 2026 Feb 1;21(2):577-587. doi: 10.4103/NRR.NRR-D-24-00846. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885674 Free PMC article.
References
-
- Freskgård P. O., Urich E., Antibody therapies in CNS diseases. Neuropharmacology 120, 38–55 (2017). - PubMed
-
- Kucher K., et al. , First-in-man intrathecal application of neurite growth-promoting anti-nogo- a antibodies in acute spinal cord injury. Neurorehabil. Neural Repair 32, 578–589 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

