Chemical Stability and Characterization of Degradation Products of Blends of 1-(2-Hydroxyethyl)pyrrolidine and 3-Amino-1-propanol
- PMID: 36649443
- PMCID: PMC9838088
- DOI: 10.1021/acs.iecr.2c03068
Chemical Stability and Characterization of Degradation Products of Blends of 1-(2-Hydroxyethyl)pyrrolidine and 3-Amino-1-propanol
Abstract
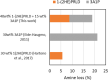
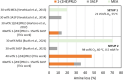
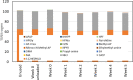
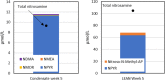
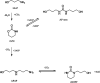
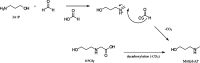
Aqueous amine solvents are used to capture CO2 from various flue gas sources. In this work, the chemical stability of a blend of 3-amino-1-propanol (3A1P) and 1-(2-hydroxyethyl)pyrrolidine [1-(2HE)PRLD] was studied. The chemical stability tests were conducted both in batch and cycled systems using various oxygen and NOx concentrations, additives (iron), and temperatures. In the thermal degradation experiments with CO2 present, the blend was more stable than the primary amines [(3A1P or monoethanolamine (MEA)] but less stable than the tertiary amine 1-(2HE)PRLD alone. Similar stability was observed between MEA, 3A1P, and the blend in the batch experiments at medium oxygen concentration (21% O2) and no iron present. 1-(2HE)PRLD was more stable. However, the presence of high oxygen concentration (96% O2) and iron reduced the stability of 1-(2HE)PRLD significantly. Furthermore, in the case of the blend, the chemical stability increased with increasing promoter concentration in batch experiments. During the cyclic experiment, the amine loss for the blend was similar to what was previously observed for MEA (30 wt %) under the same conditions. A thorough mapping of degradation compounds in the solvent and condensate samples resulted in the identification and quantification of 30 degradation compounds. The major components in batch and cycled experiments varied somewhat, as expected. In the cyclic experiments, the major components were ammonia, 3-(methylamino)-1-propanol (methyl-AP), N,N'-bis(3-hydroxypropyl)-urea (AP-urea), pyrrolidine, formic acid (formate), and N-(3-hydroxypropyl)-glycine (HPGly). Finally, in this paper, formation pathways for the eight degradation compounds (1,3-oxazinan-2-one, AP-urea, 3-[(3-aminopropyl)amino]-1-propanol, tetrahydro-1-(3-hydroxypropyl)-2(1H)-pyrimidinone, methyl-AP, N-(3-hydroxypropyl)-formamide, N-(3-hydroxypropyl)-β-alanine, and HPGly) are suggested.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















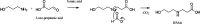

References
-
- Hartono A.; Rennemo R.; Awais M.; Vevelstad S. J.; Brakstad O. G.; Kim I.; Knuutila H. K. Characterization of 2-piperidineethanol and 1-(2-hydroxyethyl)pyrrolidine as strong bicarbonate forming solvents for CO2 capture. Int. J. Greenhouse Gas Control 2017, 63, 260–271. 10.1016/j.ijggc.2017.05.021. - DOI
-
- Hartono A.; Vevelstad S. J.; Ciftja A.; Knuutila H. K. Screening of strong bicarbonate forming solvents for CO2 capture. Int. J. Greenhouse Gas Control 2017, 58, 201–211. 10.1016/j.ijggc.2016.12.018. - DOI
-
- Hartono A.; Vevelstad S. J.; Kim I.; Rennemo R.; Knuutila H. K. Promoted Strong Bicarbonate Forming Solvents for CO2 Capture. Energy Procedia 2017, 114, 1794–1802. 10.1016/j.egypro.2017.03.1307. - DOI
-
- Kvamsdal H. M.; Kim I.; Van Os P.; Pevida C.; Hägg M.-B.; Brown J.; Robinson L.; Feron P. HiPerCap: A New FP7 Project for Development and Assessment of Novel and Emerging Post-combustion CO2 Capture Technologies. Energy Procedia 2014, 63, 6166–6172. 12th International Conference on Greenhouse Gas Control Technologies, GHGT-1210.1016/j.egypro.2014.11.648. - DOI
-
- Hartono A.; Vevelstad S. J.; Ciftja A.; Knuutila H. K. Screening of strong bicarbonate forming solvents for CO2 capture. Int. J. Greenhouse Gas Control 2017, 58, 201–211. 10.1016/j.ijggc.2016.12.018. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
