Nuclear Methods for Immune Cell Imaging: Bridging Molecular Imaging and Individualized Medicine
- PMID: 36649445
- PMCID: PMC9858352
- DOI: 10.1161/CIRCIMAGING.122.014067
Nuclear Methods for Immune Cell Imaging: Bridging Molecular Imaging and Individualized Medicine
Abstract
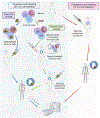
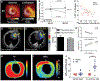
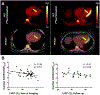
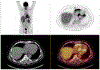
Inflammation is a key mechanistic contributor to the progression of cardiovascular disease, from atherosclerosis through ischemic injury and overt heart failure. Recent evidence has identified specific roles of immune cell subpopulations in cardiac pathogenesis that diverges between individual patients. Nuclear imaging approaches facilitate noninvasive and serial quantification of inflammation severity, offering the opportunity to predict eventual outcome, stratify patient risk, and guide novel targeted molecular therapies against specific leukocyte subpopulations. Here, we will discuss the established and emerging nuclear imaging methods to label and track exogenous and endogenous immune cells, with a particular focus on clinical situations in which targeted molecular inflammation imaging would be advantageous. The expanding options for imaging inflammation provide the foundation to bridge between molecular imaging and individual therapy.
Keywords: heart failure; inflammation; magnetic resonance imaging; myocardial infarction; positron emission tomography.
Figures


References
-
- Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res. 2019;124:263–278. doi: 10.1161/circresaha.118.314028 - DOI - PMC - PubMed
-
- Ni SH, Xu JD, Sun SN, Li Y, Zhou Z, Li H, Liu X, Deng JP, Huang YS, Chen ZX, Feng WJ, Wang JJ, Xian SX, Yang ZQ, Wang S, Wang LJ, Lu L. Single-cell transcriptomic analyses of cardiac immune cells reveal that Rel-driven CD72-positive macrophages induce cardiomyocyte injury. Cardiovasc Res. 2022;118:1303–1320. doi: 10.1093/cvr/cvab193 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

