Imaging Methods: Magnetic Resonance Imaging
- PMID: 36649450
- PMCID: PMC7614460
- DOI: 10.1161/CIRCIMAGING.122.014068
Imaging Methods: Magnetic Resonance Imaging
Abstract
Myocardial inflammation occurs following activation of the cardiac immune system, producing characteristic changes in the myocardial tissue. Cardiovascular magnetic resonance is the non-invasive imaging gold standard for myocardial tissue characterization, and is able to detect image signal changes that may occur resulting from inflammation, including edema, hyperemia, capillary leak, necrosis, and fibrosis. Conventional cardiovascular magnetic resonance for the detection of myocardial inflammation and its sequela include T2-weighted imaging, parametric T1- and T2-mapping, and gadolinium-based contrast-enhanced imaging. Emerging techniques seek to image several parameters simultaneously for myocardial tissue characterization, and to depict subtle immune-mediated changes, such as immune cell activity in the myocardium and cardiac cell metabolism. This review article outlines the underlying principles of current and emerging cardiovascular magnetic resonance methods for imaging myocardial inflammation.
Keywords: cardiac imaging techniques; immune system; inflammation; magnetic resonance imaging.
Figures


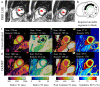
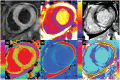
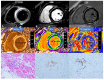
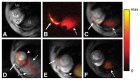
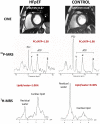
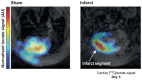
References
-
- Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nature Reviews Cardiology. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. - DOI - PMC - PubMed
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648.:2648a-2648d. doi: 10.1093/eurheartj/eht210. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

