Effects of Dapagliflozin on EChOcardiographic Measures of CarDiac StructurE and Function in Patients with Chronic Kidney Disease: The DECODE-CKD Trial
- PMID: 36649484
- PMCID: PMC10103327
- DOI: 10.34067/KID.0006982022
Effects of Dapagliflozin on EChOcardiographic Measures of CarDiac StructurE and Function in Patients with Chronic Kidney Disease: The DECODE-CKD Trial
Abstract
Key Points:
SGLT2 inhibitors (SGLT2i) exert cardioprotective effects in patients with CKD through unknown mechanisms.
DECODE-CKD is the first randomized controlled trial (RCT) to evaluate the effects of SGLT2i on cardiac structure and function in patients with CKD.
Background: SGLT2 inhibitors, originally developed as glucose-lowering agents for treatment of type 2 diabetes, have been shown to have cardio- and kidney-protective effects among CKD patients with and without diabetes. However, the mechanisms remain largely unknown.
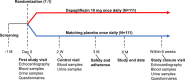
Methods: Dapagliflozin on EChOcardiographic Measures of CarDiac StructurE and Function in Patients with Chronic Kidney Disease (DECODE-CKD) is an investigator-initiated, prospective, single-center, randomized, placebo-controlled trial evaluating the effects of 6 months of treatment with 10 mg of dapagliflozin compared with placebo on cardiac structure and function in 222 adults with CKD.
Results: The primary objective was to assess whether dapagliflozin improves left ventricular mass index. Secondary and exploratory end points include changes in cardiac and kidney markers, quality of life, depressive symptoms, and cognitive function.
Conclusions: This is the first study to address the effects of SGLT2 inhibitors on cardiac structure and function in patients with CKD. The results will provide valuable insights into the mechanisms underlying the cardioprotective benefits of SGLT2 inhibitors in patients with CKD.
Clinical Trial registry name and registration number:
Conflict of interest statement
K.V. Bartholdy reports the following: Research Funding: Danish Cardiovascular Academy and Novo Nordisk Foundation. T. Biering-Sørensen reports the following: Research Funding: AstraZeneca, GE Healthcare, Novo Nordisk, and Sanofi Pasteur; Honoraria: Bayer, GSK, Novartis, and Sanofi Pasteur; and Advisory or Leadership Role: Amgen, GSK, and Sanofi Pasteur. I. Bressendorff reports the following: Research Funding: Novo Nordisk Foundation; and Speakers Bureau: Bayer AG. J. Christensen reports the following: Research Funding: Novo Nordisk Fonden. D. Hansen reports the following: Research Funding: Astra Zeneca A/S, Bayer A/S, Boehringer Ingelheim, Gedeon Richter, Kappa Bioscience AS, and Vifor Pharma; Advisory or Leadership Role: Pharmacosmos; and Speakers Bureau: Astra-Zeneca A/S and UCB Nordic. R. Haynes reports the following: Research Funding: Boehringer-Ingelheim and Novartis. J. Jensen reports the following: Honoraria: Boehringer Ingelheim; Advisory or Leadership Role: AstraZeneca, Boehringer Ingelheim; and Other Interests or Relationships: The CARDIOHGH Foundation and The Danish Heart Foundation. L. Køber reports the following: Honoraria: Speakers honorarium from AstraZeneca, Bayer, Boehringer, and Novartis. F. Persson reports the following: Consultancy: Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and Sanofi; Research Funding: Amgen, Astra Zeneca, Boehringer Ingelheim, and Novo Nordisk; and Honoraria: Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and Sanofi. P. Rossing reports the following: Research Funding: AstraZeneca, Bayer, and Novo Nordisk; Honoraria: all honoraria to institution, AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and Advisory or Leadership Role: Astellas, Astra Zeneca, Bayer, MSD Gilead all honoraria to institution, and Novo nordisk. M. Schou reports the following: Speakers Bureau: lecture fees from Asra Zeneca, Bohringer, Novartis, and Novo. K.G. Skaarup reports the following: Advisory or Leadership Role: Sanofi Pasteur. S. Solomon reports the following: Consultancy: Abbott, Action, Akros, Alnylam, American Regent, Amgen, Anacardio, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, BMS, Cardiac Dimensions, Cardior, Cardurion, CellProThera, Corvia, Cytokinetics, Daiichi-Sankyo, Dinaqor, GSK, Janssen, Lexicon, Lilly, Merck, Moderna, Myokardia, Novartis, Puretech Health, Quantum Genomics, Roche, Sanofi-Pasteur, Sarepta, Tenaya, Theracos, and Tremeau; Research Funding: Actelion, Alnylam, Amgen, AstraZeneca, Bayer, Bellerophon, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, Neurotronik, NIH/NHLBI, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and Advisory or Leadership Role:
Figures
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Sarnak MJ Levey AS Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154-2169. doi: 10.1161/01.cir.0000095676.90936.80 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical