Kindlin-3 deficiency leads to impaired erythropoiesis and erythrocyte cytoskeleton
- PMID: 36649586
- PMCID: PMC10182306
- DOI: 10.1182/bloodadvances.2022008498
Kindlin-3 deficiency leads to impaired erythropoiesis and erythrocyte cytoskeleton
Abstract
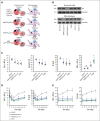
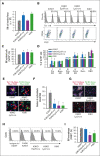
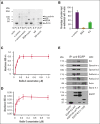
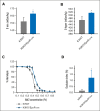
Kindlin-3 (K3) is critical for the activation of integrin adhesion receptors in hematopoietic cells. In humans and mice, K3 deficiency is associated with impaired immunity and bone development, bleeding, and aberrant erythrocyte shape. To delineate how K3 deficiency (K3KO) contributes to anemia and misshaped erythrocytes, mice deficient in erythroid (K3KO∖EpoR-cre) or myeloid cell K3 (K3KO∖Lyz2cre), knockin mice expressing mutant K3 (Q597W598 to AA) with reduced integrin-activation function (K3KI), and control wild-type (WT) K3 mice were studied. Both K3-deficient strains and K3KI mice showed anemia at baseline, reduced response to erythropoietin stimulation, and compromised recovery after phenylhydrazine (PHZ)-induced hemolytic anemia as compared with K3WT. Erythroid K3KO and K3 (Q597W598 to AA) showed arrested erythroid differentiation at proerythroblast stage, whereas macrophage K3KO showed decreased erythroblast numbers at all developmental stages of terminal erythroid differentiation because of reduced erythroblastic island (EBI) formation attributable to decreased expression and activation of erythroblast integrin α4β1 and macrophage αVβ3. Peripheral blood smears of K3KO∖EpoR-cre mice, but not of the other mouse strains, showed numerous aberrant tear drop-shaped erythrocytes. K3 deficiency in these erythrocytes led to disorganized actin cytoskeleton, reduced deformability, and increased osmotic fragility. Mechanistically, K3 directly interacted with F-actin through an actin-binding site K3-LK48. Taken together, these findings document that erythroid and macrophage K3 are critical contributors to erythropoiesis in an integrin-dependent manner, whereas F-actin binding to K3 maintains the membrane cytoskeletal integrity and erythrocyte biconcave shape. The dual function of K3 in erythrocytes and in EBIs establish an important functional role for K3 in normal erythroid function.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

