Spatially selective stimulation of the pig vagus nerve to modulate target effect versus side effect
- PMID: 36649655
- PMCID: PMC10339030
- DOI: 10.1088/1741-2552/acb3fd
Spatially selective stimulation of the pig vagus nerve to modulate target effect versus side effect
Abstract
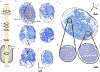
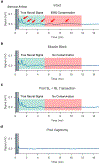
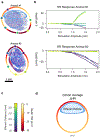
Electrical stimulation of the cervical vagus nerve using implanted electrodes (VNS) is FDA-approved for the treatment of drug-resistant epilepsy, treatment-resistant depression, and most recently, chronic ischemic stroke rehabilitation. However, VNS is critically limited by the unwanted stimulation of nearby neck muscles-a result of non-specific stimulation activating motor nerve fibers within the vagus. Prior studies suggested that precise placement of small epineural electrodes can modify VNS therapeutic effects, such as cardiac responses. However, it remains unclear if placement can alter the balance between intended effect and limiting side effect. We used an FDA investigational device exemption approved six-contact epineural cuff to deliver VNS in pigs and quantified how epineural electrode location impacts on- and off-target VNS activation. Detailed post-mortem histology was conducted to understand how the underlying neuroanatomy impacts observed functional responses. Here we report the discovery and characterization of clear neuroanatomy-dependent differences in threshold and saturation for responses related to both effect (change in heart rate) and side effect (neck muscle contractions). The histological and electrophysiological data were used to develop and validate subject-specific computation models of VNS, creating a well-grounded quantitative framework to optimize electrode location-specific activation of nerve fibers governing intended effect versus unwanted side effect.
Keywords: VNS; effect vs side effect; neuromodulation; vagus nerve.
Creative Commons Attribution license.
Conflict of interest statement
Conflicts of Interest
JW and KL are scientific board members and have stock interests in NeuroOne Medical Inc., a company developing next-generation epilepsy monitoring devices. JW also has an equity interest in NeuroNexus technology Inc., a company that supplies electrophysiology equipment and multichannel probes to the neuroscience research community. KL is also a paid member of the scientific advisory board of Cala Health, Blackfynn, Abbott, and Battelle. KL also is a paid consultant for Galvani and Boston Scientific. KL and AS are consultants to and co-founders of Neuronoff Inc. None of these associations are directly relevant to the work presented in this manuscript.
Additionally, RV and JB are employed by LivaNova USA Inc., a vagus nerve stimulation company, and hold stock or stock options. RV and JB contributed primarily to the initial conceptualization and financial support for the program. The authorship team asserts that their conflict did not influence the collection, analysis, or interpretation of study results. The authorship team asserts that financial contributions of LivaNova USA funded study-related expenses but were not allocated for authorship.
The remaining authors declare that the research was conducted in the absence of any additional commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
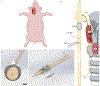




References
-
- Aristovich K, Donega M, Fjordbakk C, Tarotin I, Chapman CAR, Viscasillas J, Stathopoulou T-R, Crawford A, Chew D, Perkins J, & Holder D (2021). Model-based geometrical optimisation and in vivo validation of a spatially selective multielectrode cuff array for vagus nerve neuromodulation. Journal of Neuroscience Methods, 109079. 10.1016/j.jneumeth.2021.109079 - DOI - PubMed
-
- Hill AV. (1910). The possible effects of the aggregation of the molecules of huemoglobin on its dissociation curves. The Journal of Physiology, 40(Suppl)(PROCEEDINGS OF THE PHYSIOLOGICAL SOCIETY: January 22, 1910), iv–vii.
-
- Baumgart JP, Zhou Z-Y, Hara M, Cook DC, Hoppa MB, Ryan TA, & Hemmings HC (2015). Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proceedings of the National Academy of Sciences of the United States of America, 112(38), 11959–11964. 10.1073/pnas.1500525112 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
