Conformational exchange divergence along the evolutionary pathway of eosinophil-associated ribonucleases
- PMID: 36649708
- PMCID: PMC9992247
- DOI: 10.1016/j.str.2022.12.011
Conformational exchange divergence along the evolutionary pathway of eosinophil-associated ribonucleases
Abstract
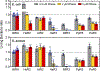
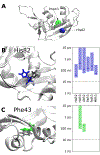
The evolutionary role of conformational exchange in the emergence and preservation of function within structural homologs remains elusive. While protein engineering has revealed the importance of flexibility in function, productive modulation of atomic-scale dynamics has only been achieved on a finite number of distinct folds. Allosteric control of unique members within dynamically diverse structural families requires a better appreciation of exchange phenomena. Here, we examined the functional and structural role of conformational exchange within eosinophil-associated ribonucleases. Biological and catalytic activity of various EARs was performed in parallel to mapping their conformational behavior on multiple timescales using NMR and computational analyses. Despite functional conservation and conformational seclusion to a specific domain, we show that EARs can display similar or distinct motional profiles, implying divergence rather than conservation of flexibility. Comparing progressively more distant enzymes should unravel how this subfamily has evolved new functions and/or altered their behavior at the molecular level.
Keywords: CEST; CPMG; Markov State Model; NMR relaxation; antibacterial activity; conformational exchange; enzyme dynamics; intrinsically disordered proteins; molecular dynamics simulations; ribonucleases.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests Pratul K. Agarwal is the founder of the company Arium BioLabs, LLC.
Figures
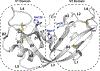

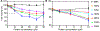



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

