Vasopressin V1a receptor and oxytocin receptor regulate murine sperm motility differently
- PMID: 36650057
- PMCID: PMC9846835
- DOI: 10.26508/lsa.202201488
Vasopressin V1a receptor and oxytocin receptor regulate murine sperm motility differently
Abstract
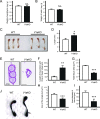
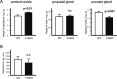
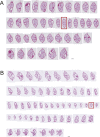
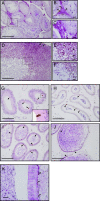
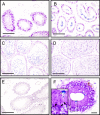
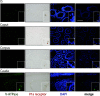
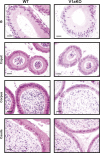
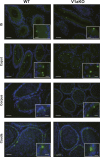
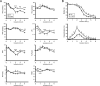
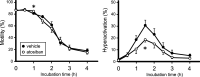
Specific receptors for the neurohypophyseal hormones, arginine vasopressin (AVP) and oxytocin, are present in the male reproductive organs. However, their exact roles remain unknown. To elucidate the physiological functions of pituitary hormones in male reproduction, this study first focused on the distribution and function of one of the AVP receptors, V1a. In situ hybridization analysis revealed high expression of the Avpr1a in Leydig cells of the testes and narrow/clear cells in the epididymis, with the expression pattern differing from that of the oxytocin receptor (OTR). Notably, persistent motility and highly proportional hyperactivation were observed in spermatozoa from V1a receptor-deficient mice. In contrast, OTR blocking by antagonist atosiban decreased hyperactivation rate. Furthermore, AVP stimulation could alter the extracellular pH mediated by the V1a receptor. The results highlight the crucial role of neurohypophyseal hormones in male reproductive physiology, with potential contradicting roles of V1a and OTR in sperm maturation. Our findings suggest that V1a receptor antagonists are potential therapeutic drugs for male infertility.
© 2023 Tsuchiya et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors.Br J Pharmacol. 2008 Sep;155(1):118-26. doi: 10.1038/bjp.2008.226. Epub 2008 Jun 16. Br J Pharmacol. 2008. PMID: 18552879 Free PMC article.
-
The role of vasopressin V1A and oxytocin OTR receptors in protective effects of arginine vasopressin against H2O2-induced oxidative stress in H9C2 cells.Arch Physiol Biochem. 2022 Jun;128(3):830-835. doi: 10.1080/13813455.2020.1729816. Epub 2020 Mar 6. Arch Physiol Biochem. 2022. PMID: 32141340
-
Binding affinities of oxytocin, vasopressin and Manning compound at oxytocin and V1a receptors in male Syrian hamster brains.J Neuroendocrinol. 2020 Jul;32(7):e12882. doi: 10.1111/jne.12882. Epub 2020 Jul 14. J Neuroendocrinol. 2020. PMID: 32662552 Free PMC article.
-
Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: Implications for cross-system signaling.Front Neuroendocrinol. 2019 Apr;53:100737. doi: 10.1016/j.yfrne.2019.02.001. Epub 2019 Feb 10. Front Neuroendocrinol. 2019. PMID: 30753840 Free PMC article. Review.
-
Potential use of oxytocin and vasopressin V1a antagonists in the treatment of preterm labour and primary dysmenorrhoea.Adv Exp Med Biol. 1995;395:595-600. Adv Exp Med Biol. 1995. PMID: 8714023 Review.
Cited by
-
Enhancement of rat spermatozoal hyperactivation by progesterone.J Reprod Dev. 2023 Oct 20;69(5):279-290. doi: 10.1262/jrd.2023-040. Epub 2023 Sep 10. J Reprod Dev. 2023. PMID: 37690839 Free PMC article.
-
Mechanism of salidroside promoting testosterone secretion induced by H2O2 in TM3 Leydig cells based on metabolomics and network pharmacology.Front Chem. 2025 Feb 27;13:1544876. doi: 10.3389/fchem.2025.1544876. eCollection 2025. Front Chem. 2025. PMID: 40084278 Free PMC article.
References
-
- Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, Serradeil-Le Gal C, Steinwall M (1999) Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol 106: 1047–1053. 10.1111/j.1471-0528.1999.tb08112.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
