Mechanisms That Underlie Expression of Estradiol-Induced Excitatory Synaptic Potentiation in the Hippocampus Differ between Males and Females
- PMID: 36650060
- PMCID: PMC9987570
- DOI: 10.1523/JNEUROSCI.2080-19.2023
Mechanisms That Underlie Expression of Estradiol-Induced Excitatory Synaptic Potentiation in the Hippocampus Differ between Males and Females
Abstract
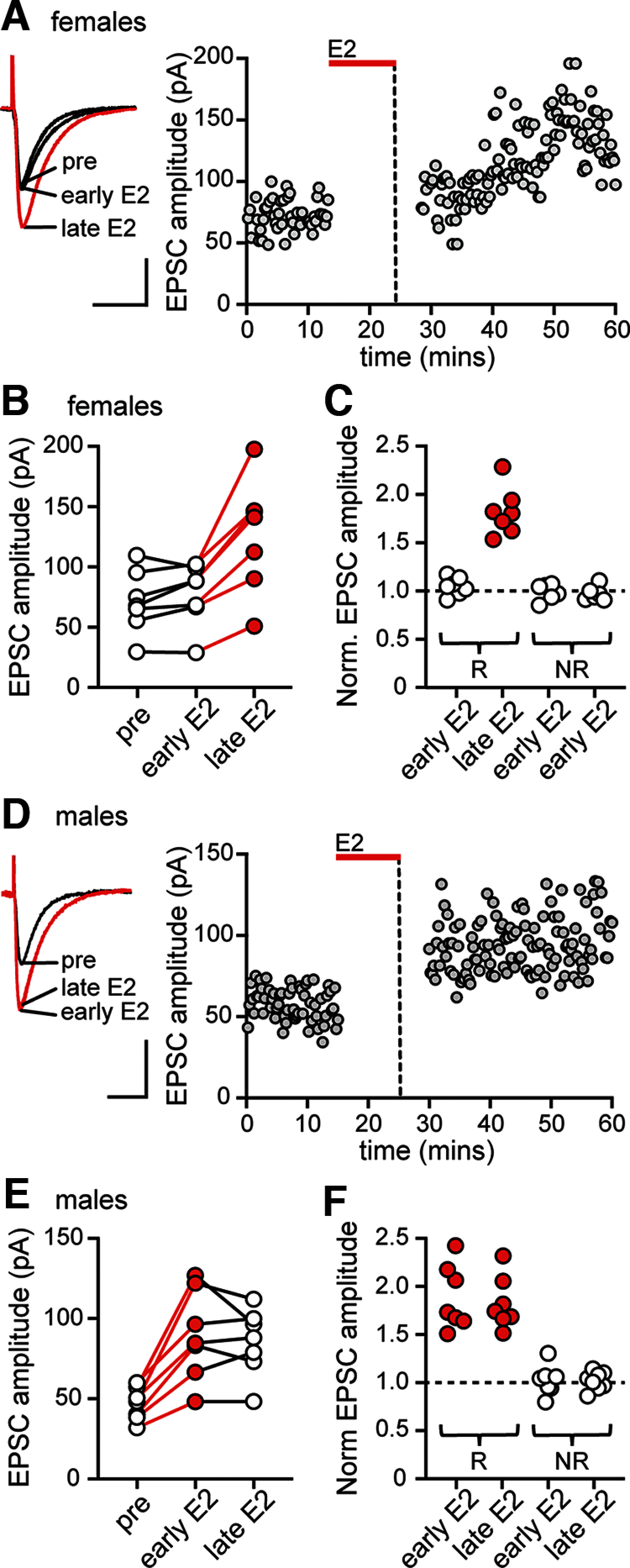
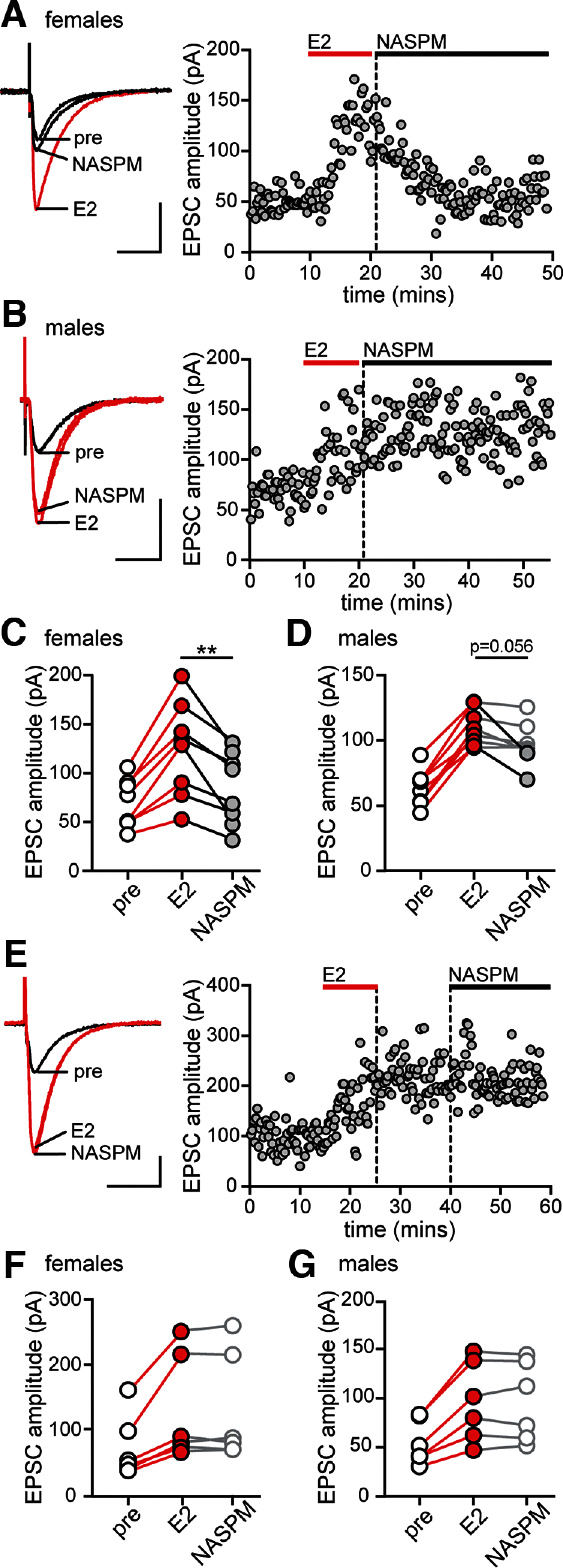
17β-estradiol (E2) is synthesized in the hippocampus of both sexes and acutely potentiates excitatory synapses in each sex. Previously, we found that the mechanisms for initiation of E2-induced synaptic potentiation differ between males and females, including in the molecular signaling involved. Here, we used electrical stimulation and two-photon glutamate uncaging in hippocampal slices from adult male and female rats to investigate whether the downstream consequences of distinct molecular signaling remain different between the sexes or converge to the same mechanism(s) of expression of potentiation. This showed that synaptic activity is necessary for expression of E2-induced potentiation in females but not males, which paralleled a sex-specific requirement in females for calcium-permeable AMPARs (cpAMPARs) to stabilize potentiation. Nonstationary fluctuation analysis of two-photon evoked unitary synaptic currents showed that the postsynaptic component of E2-induced potentiation occurs either through an increase in AMPAR conductance or in nonconductive properties of AMPARs (number of channels × open probability) and never both at the same synapse. In females, most synapses (76%) were potentiated via increased AMPAR conductance, whereas in males, more synapses (60%) were potentiated via an increase in nonconductive AMPAR properties. Inhibition of cpAMPARs eliminated E2-induced synaptic potentiation in females, whereas some synapses in males were unaffected by cpAMPAR inhibition; these synapses in males potentiated exclusively via increased AMPAR nonconductive properties. This sex bias in expression mechanisms of E2-induced synaptic potentiation underscores the concept of latent sex differences in mechanisms of synaptic plasticity in which the same outcome in each sex is achieved through distinct underlying mechanisms.SIGNIFICANCE STATEMENT Estrogens are synthesized in the brains of both sexes and potentiate excitatory synapses to the same degree in each sex. Despite this apparent similarity, the molecular signaling that initiates estrogen-induced synaptic potentiation differs between the sexes. Here we show that these differences extend to the mechanisms of expression of synaptic potentiation and result in distinct patterns of postsynaptic neurotransmitter receptor modulation in each sex. Such latent sex differences, in which the same outcome is achieved through distinct underlying mechanisms in males versus females, indicate that molecular mechanisms targeted for drug development may differ between the sexes even in the absence of an overt sex difference in behavior or disease.
Keywords: AMPA receptor; neurosteroid; plasticity; sex difference; synapse.
Copyright © 2023 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
17β-Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females.J Neurosci. 2016 Mar 2;36(9):2677-90. doi: 10.1523/JNEUROSCI.4437-15.2016. J Neurosci. 2016. PMID: 26937008 Free PMC article.
-
Latent Sex Differences in Molecular Signaling That Underlies Excitatory Synaptic Potentiation in the Hippocampus.J Neurosci. 2019 Feb 27;39(9):1552-1565. doi: 10.1523/JNEUROSCI.1897-18.2018. Epub 2018 Dec 21. J Neurosci. 2019. PMID: 30578341 Free PMC article.
-
Memory-Related Synaptic Plasticity Is Sexually Dimorphic in Rodent Hippocampus.J Neurosci. 2018 Sep 12;38(37):7935-7951. doi: 10.1523/JNEUROSCI.0801-18.2018. Epub 2018 Aug 1. J Neurosci. 2018. PMID: 30209204 Free PMC article.
-
Modulation of synaptic plasticity by brain estrogen in the hippocampus.Biochim Biophys Acta. 2010 Oct;1800(10):1030-44. doi: 10.1016/j.bbagen.2009.11.002. Epub 2009 Nov 10. Biochim Biophys Acta. 2010. PMID: 19909788 Review.
-
Sexual neurosteroids and synaptic plasticity in the hippocampus.Brain Res. 2015 Sep 24;1621:162-9. doi: 10.1016/j.brainres.2014.10.033. Epub 2014 Oct 28. Brain Res. 2015. PMID: 25452021 Review.
Cited by
-
Dendritic, delayed, stochastic CaMKII activation in behavioural time scale plasticity.Nature. 2024 Nov;635(8037):151-159. doi: 10.1038/s41586-024-08021-8. Epub 2024 Oct 9. Nature. 2024. PMID: 39385027 Free PMC article.
-
Inhibition of Estradiol Signaling in the Basolateral Amygdala Impairs Extinction Memory Recall for Heroin-Conditioned Cues in a Sex-Specific Manner.Neuroendocrinology. 2024;114(3):207-222. doi: 10.1159/000534647. Epub 2023 Oct 17. Neuroendocrinology. 2024. PMID: 37848008 Free PMC article.
-
Estrogen receptor beta signaling enhances extinction memory recall for heroin-conditioned cues in a sex- and region-specific manner.Transl Psychiatry. 2024 Jul 12;14(1):283. doi: 10.1038/s41398-024-03001-y. Transl Psychiatry. 2024. PMID: 38997258 Free PMC article.
-
Shorter Infantile Amnesia in Females: Important Implications for the Next Generation.Cells. 2025 Feb 28;14(5):354. doi: 10.3390/cells14050354. Cells. 2025. PMID: 40072083 Free PMC article. Review.
-
G protein-coupled estrogen receptor (GPER) in the dorsal hippocampus regulates memory consolidation in gonadectomized male mice, likely via different signaling mechanisms than in female mice.Horm Behav. 2024 May;161:105516. doi: 10.1016/j.yhbeh.2024.105516. Epub 2024 Mar 1. Horm Behav. 2024. PMID: 38428223 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
