A RIPK3-independent role of MLKL in suppressing parthanatos promotes immune evasion in hepatocellular carcinoma
- PMID: 36650126
- PMCID: PMC9845215
- DOI: 10.1038/s41421-022-00504-0
A RIPK3-independent role of MLKL in suppressing parthanatos promotes immune evasion in hepatocellular carcinoma
Abstract
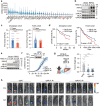
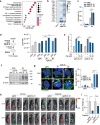
Mixed lineage kinase domain-like (MLKL) is widely accepted as an executioner of necroptosis, in which MLKL mediates necroptotic signaling and triggers cell death in a receptor-interacting protein kinase 3 (RIPK3)-dependent manner. Recently, it is increasingly noted that RIPK3 is intrinsically silenced in hepatocytes, raising a question about the role of MLKL in hepatocellular carcinoma (HCC). This study reports a previously unrecognized role of MLKL in regulating parthanatos, a programmed cell death distinct from necroptosis. In HCC cells with intrinsic RIPK3 deficiency, knockout of MLKL impedes the orthotopic tumor growth, activates the anti-tumor immune response and enhances the therapeutic effect of immune checkpoint blockade in syngeneic HCC tumor models. Mechanistically, MLKL is required for maintaining the endoplasmic reticulum (ER)-mitochondrial Mg2+ dynamics in HCC cells. MLKL deficiency restricts ER Mg2+ release and mitochondrial Mg2+ uptake, leading to ER dysfunction and mitochondrial oxidative stress, which together confer increased susceptibility to metabolic stress-induced parthanatos. Importantly, pharmacological inhibition of poly(ADP-ribose) polymerase to block parthanatos restores the tumor growth and immune evasion in MLKL-knockout HCC tumors. Together, our data demonstrate a new RIPK3-independent role of MLKL in regulating parthanatos and highlight the role of MLKL in facilitating immune evasion in HCC.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

