Harnessing gut microbes for glycan detection and quantification
- PMID: 36650134
- PMCID: PMC9845299
- DOI: 10.1038/s41467-022-35626-2
Harnessing gut microbes for glycan detection and quantification
Abstract
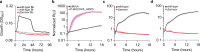
Glycans facilitate critical biological functions and control the mammalian gut microbiota composition by supplying differentially accessible nutrients to distinct microbial subsets. Therefore, identifying unique glycan substrates that support defined microbial populations could inform therapeutic avenues to treat diseases via modulation of the gut microbiota composition and metabolism. However, examining heterogeneous glycan mixtures for individual microbial substrates is hindered by glycan structural complexity and diversity, which presents substantial challenges to glycomics approaches. Fortuitously, gut microbes encode specialized sensor proteins that recognize unique glycan structures and in-turn activate predictable, specific, and dynamic transcriptional responses. Here, we harness this microbial machinery to indicate the presence and abundance of compositionally similar, yet structurally distinct glycans, using a transcriptional reporter we develop. We implement these tools to examine glycan mixtures, isolate target molecules for downstream characterization, and quantify the recovered products. We assert that this toolkit could dramatically enhance our understanding of the mammalian intestinal environment and identify host-microbial interactions critical for human health.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

