Bottom-up evolution of perovskite clusters into high-activity rhodium nanoparticles toward alkaline hydrogen evolution
- PMID: 36650135
- PMCID: PMC9845238
- DOI: 10.1038/s41467-023-35783-y
Bottom-up evolution of perovskite clusters into high-activity rhodium nanoparticles toward alkaline hydrogen evolution
Abstract
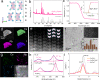
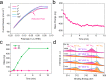
Self-reconstruction has been considered an efficient means to prepare efficient electrocatalysts in various energy transformation process for bond activation and breaking. However, developing nano-sized electrocatalysts through complete in-situ reconstruction with improved activity remains challenging. Herein, we report a bottom-up evolution route of electrochemically reducing Cs3Rh2I9 halide-perovskite clusters on N-doped carbon to prepare ultrafine Rh nanoparticles (~2.2 nm) with large lattice spacings and grain boundaries. Various in-situ and ex-situ characterizations including electrochemical quartz crystal microbalance experiments elucidate the Cs and I extraction and Rh reduction during the electrochemical reduction. These Rh nanoparticles from Cs3Rh2I9 clusters show significantly enhanced mass and area activity toward hydrogen evolution reaction in both alkaline and chlor-alkali electrolyte, superior to liquid-reduced Rh nanoparticles as well as bulk Cs3Rh2I9-derived Rh via top-down electro-reduction transformation. Theoretical calculations demonstrate water activation could be boosted on Cs3Rh2I9 clusters-derived Rh nanoparticles enriched with multiply sites, thus smoothing alkaline hydrogen evolution.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- McCrum IT, Koper MTM. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy. 2020;5:891–899. doi: 10.1038/s41560-020-00710-8. - DOI
-
- Hu C, Zhang L, Gong J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019;12:2620–2645. doi: 10.1039/C9EE01202H. - DOI
-
- Peugeot A, et al. Benchmarking of oxygen evolution catalysts on porous nickel supports. Joule. 2021;5:1281–1300. doi: 10.1016/j.joule.2021.03.022. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

