Tumor-specific intracellular delivery: peptide-guided transport of a catalytic toxin
- PMID: 36650239
- PMCID: PMC9845330
- DOI: 10.1038/s42003-022-04385-7
Tumor-specific intracellular delivery: peptide-guided transport of a catalytic toxin
Abstract
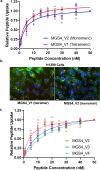
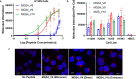
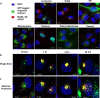
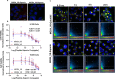
There continues to be a need for cancer-specific ligands that can deliver a wide variety of therapeutic cargos. Ligands demonstrating both tumor-specificity and the ability to mediate efficient cellular uptake of a therapeutic are critical to expand targeted therapies. We previously reported the selection of a peptide from a peptide library using a non-small cell lung cancer (NSCLC) cell line as the target. Here we optimize our lead peptide by a series of chemical modifications including truncations, N-terminal capping, and changes in valency. The resultant 10 amino acid peptide has an affinity of <40 nM on four different NSCLC cell lines as a monomer and is stable in human serum for >48 h. The peptide rapidly internalizes upon cell binding and traffics to the lysosome. The peptide homes to a tumor in an animal model and is retained up to 72 h. Importantly, we demonstrate that the peptide can deliver the cytotoxic protein saporin specifically to cancer cells in vitro and in vivo, resulting in an effective anticancer agent.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- American Cancer Society: Cancer Facts & Figures 2022, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

