Clinical spectrum and currently available treatment of type I interferonopathy Aicardi-Goutières syndrome
- PMID: 36650407
- PMCID: PMC10258176
- DOI: 10.1007/s12519-022-00679-2
Clinical spectrum and currently available treatment of type I interferonopathy Aicardi-Goutières syndrome
Abstract
Background: Aicardi-Goutières syndrome (AGS) is a genetically determined disorder with a variable phenotype. Since the original description of AGS, advances in gene sequencing techniques have resulted in a significant broadening of the phenotypic spectrum associated with AGS genes, and new clinical pictures have emerged beyond the classic presentation. The aim of this review is to provide a comprehensive analysis of the clinical spectrum of AGS and report currently available treatments and new immunosuppressive strategies.
Data sources: Literature reviews and original research articles were collected from databases, including PubMed and ClinicalTrials.gov. Relevant articles about AGS were included.
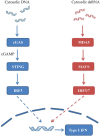
Results: The involvement of the nervous system certainly represents the major cause of mortality and morbidity in AGS patients. However, other clinical manifestations, such as chilblains, hepatosplenomegaly, and hematological disturbances, may lead to the diagnosis and considerably impact the prognosis and overall quality of life of these patients. Therapeutic approaches of AGS are limited to interventions aimed at specific symptoms and the management of multiple comorbidities. However, advances in understanding the pathogenesis of AGS could open new and more effective therapies.
Conclusions: The over-activation of innate immunity due to upregulated interferon production plays a critical role in AGS, leading to multi-organ damage with the main involvement of the central nervous system. To date, there is no specific and effective treatment for AGS. New drugs specifically targeting the interferon pathway may bring new hope to AGS patients.
Keywords: Aicardi–Goutières syndrome; Immunosuppressive drugs; Interferon-α; Neuroinflammation; Systemic lupus erythematosus.
© 2023. The Author(s).
Conflict of interest statement
No financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
Figures
References
-
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GMA, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet Part A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

