Payload diversification: a key step in the development of antibody-drug conjugates
- PMID: 36650546
- PMCID: PMC9847035
- DOI: 10.1186/s13045-022-01397-y
Payload diversification: a key step in the development of antibody-drug conjugates
Abstract
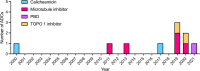
Antibody-drug conjugates (ADCs) is a fast moving class of targeted biotherapeutics that currently combines the selectivity of monoclonal antibodies with the potency of a payload consisting of cytotoxic agents. For many years microtubule targeting and DNA-intercalating agents were at the forefront of ADC development. The recent approval and clinical success of trastuzumab deruxtecan (Enhertu®) and sacituzumab govitecan (Trodelvy®), two topoisomerase 1 inhibitor-based ADCs, has shown the potential of conjugating unconventional payloads with differentiated mechanisms of action. Among future developments in the ADC field, payload diversification is expected to play a key role as illustrated by a growing number of preclinical and clinical stage unconventional payload-conjugated ADCs. This review presents a comprehensive overview of validated, forgotten and newly developed payloads with different mechanisms of action.
Keywords: Antibody–drug conjugates; Cytotoxic molecule; Payload; Topoisomerase 1 inhibitor.
© 2023. The Author(s).
Conflict of interest statement
LC, LS and WV are employees of Mablink Bioscience. CD and WV are shareholders of Mablink Bioscience.
Figures



References
-
- Bross PF, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

