Dynamic states of eIF6 and SDS variants modulate interactions with uL14 of the 60S ribosomal subunit
- PMID: 36651285
- PMCID: PMC9976893
- DOI: 10.1093/nar/gkac1266
Dynamic states of eIF6 and SDS variants modulate interactions with uL14 of the 60S ribosomal subunit
Abstract
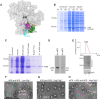
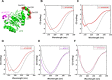
Assembly of ribosomal subunits into active ribosomal complexes is integral to protein synthesis. Release of eIF6 from the 60S ribosomal subunit primes 60S to associate with the 40S subunit and engage in translation. The dynamics of eIF6 interaction with the uL14 (RPL23) interface of 60S and its perturbation by somatic mutations acquired in Shwachman-Diamond Syndrome (SDS) is yet to be clearly understood. Here, by using a modified strategy to obtain high yields of recombinant human eIF6 we have uncovered the critical interface entailing eight key residues in the C-tail of uL14 that is essential for physical interactions between 60S and eIF6. Disruption of the complementary binding interface by conformational changes in eIF6 disease variants provide a mechanism for weakened interactions of variants with the 60S. Hydrogen-deuterium exchange mass spectrometry (HDX-MS) analyses uncovered dynamic configurational rearrangements in eIF6 induced by binding to uL14 and exposed an allosteric interface regulated by the C-tail of eIF6. Disrupting key residues in the eIF6-60S binding interface markedly limits proliferation of cancer cells, which highlights the significance of therapeutically targeting this interface. Establishing these key interfaces thus provide a therapeutic framework for targeting eIF6 in cancers and SDS.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures







References
-
- Nerurkar P., Altvater M., Gerhardy S., Schutz S., Fischer U., Weirich C., Panse V.G.. Eukaryotic ribosome assembly and nuclear export. Int. Rev. Cell Mol. Biol. 2015; 319:107–140. - PubMed
-
- Brina D., Miluzio A., Ricciardi S., Biffo S.. eIF6 anti-association activity is required for ribosome biogenesis, translational control and tumor progression. Biochim. Biophys. Acta. 2015; 1849:830–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

