Once-daily oral atogepant for the long-term preventive treatment of migraine: Findings from a multicenter, randomized, open-label, phase 3 trial
- PMID: 36651532
- PMCID: PMC10107835
- DOI: 10.1111/head.14439
Once-daily oral atogepant for the long-term preventive treatment of migraine: Findings from a multicenter, randomized, open-label, phase 3 trial
Erratum in
-
Correction to: "Once-daily oral atogepant for the long-term preventive treatment of migraine: Findings from a multicenter, randomized, open-label, phase 3 trial".Headache. 2024 Mar;64(3):329. doi: 10.1111/head.14691. Epub 2024 Feb 26. Headache. 2024. PMID: 38407505 Free PMC article. No abstract available.
Abstract
Objective: To assess long-term safety, tolerability, and efficacy of once-daily oral atogepant 60 mg in adults with migraine.
Background: Atogepant is an oral, small-molecule, calcitonin gene-related peptide receptor antagonist approved for the preventive treatment of episodic migraine.
Methods: A 52-week, multicenter, randomized, open-label trial of adults (18-80 years) with migraine. Lead-in trial completers or newly enrolled participants with 4-14 migraine days/month were enrolled and randomized (5:2) to atogepant 60 mg once daily or oral standard care (SC) migraine preventive medication. The primary objective was to evaluate the safety and tolerability of atogepant; safety assessments included treatment-emergent adverse events (TEAEs), clinical laboratory evaluations, vital signs, and Columbia-Suicide Severity Rating Scale scores. Efficacy assessments (atogepant only) included change from baseline in mean monthly migraine days (MMDs) and the proportion of participants with reductions from baseline of ≥50%, ≥75%, and 100% in MMDs.
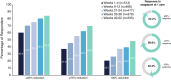
Results: The trial included 744 participants randomized to atogepant 60 mg (n = 546) or SC (n = 198). The atogepant safety population was 88.2% female (n = 479/543) with a mean (standard deviation) age of 42.5 (12.0) years. TEAEs occurred in 67.0% (n = 364/543) of participants treated with atogepant 60 mg. The most commonly reported TEAEs (≥5%) were upper respiratory tract infection (10.3%; 56/543), constipation (7.2%; 39/543), nausea (6.3%; 34/543), and urinary tract infection (5.2%; 28/543). Serious TEAEs were reported in 4.4% (24/543) for atogepant. Mean (standard error) change in MMDs for atogepant was -3.8 (0.1) for weeks 1-4 and -5.2 (0.2) at weeks 49-52. Similarly, the proportion of participants with ≥50%, ≥75%, and 100% reductions in MMDs increased from 60.4% (310/513), 37.2% (191/513), and 20.7% (106/513) at weeks 1-4 to 84.2% (282/335), 69.9% (234/335), and 48.4% (162/335), at weeks 49-52.
Conclusion: Daily use of oral atogepant 60 mg for preventive treatment of migraine during this 1-year, open-label trial was safe, well tolerated, and efficacious.
Keywords: atogepant; calcitonin gene-related peptide; gepant; migraine; migraine preventive.
© 2023 The Authors. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

