Common pathophysiology for ANXA11 disorders caused by aspartate 40 variants
- PMID: 36651622
- PMCID: PMC10014011
- DOI: 10.1002/acn3.51731
Common pathophysiology for ANXA11 disorders caused by aspartate 40 variants
Abstract
Objective: Mutations in ANXA11 cause amyotrophic lateral sclerosis (ALS) and have recently been identified as a cause of multisystem proteinopathy and adult-onset muscular dystrophy. These conditions are adult-onset diseases and result from the substitution of Aspartate 40 (Asp40) for an apolar residue in the intrinsically disordered domain (IDD) of ANXA11. Some ALS-related variants are known to affect ANXA11 IDD; however, the mechanism by which the myopathy occurs is unknown.
Methods: Genetic analysis was performed using WES-trio. For the study of variant pathogenicity, we used recombinant proteins, muscle biopsy, and fibroblasts.
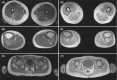
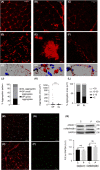
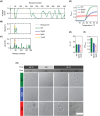
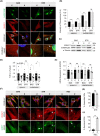
Results: Here we describe an individual with severe and rapidly progressive childhood-onset oculopharyngeal muscular dystrophy who carries a new ANXA11 variant at position Asp40 (p.Asp40Ile; c.118_119delGAinsAT). p.Asp40Ile is predicted to enhance the aggregation propensity of ANXA11 to a greater extent than other changes affecting this residue. In vitro studies using recombinant ANXA11p.Asp40Ile showed abnormal phase separation and confirmed this variant is more aggregation-prone than the ALS-associated variant ANXA11p.Asp40Gly . The study of the patient's fibroblasts revealed defects in stress granules dynamics and clearance, and muscle histopathology showed a myopathic pattern with ANXA11 protein aggregates. Super-resolution imaging showed aggregates expressed as pearl strips or large complex structures in the sarcoplasm, and as layered subsarcolemmal chains probably reflecting ANXA11 multifunctionality.
Interpretation: We demonstrate common pathophysiology for disorders associated with ANXA11 Asp40 allelic variants. Clinical phenotypes may result from different deleterious impacts of variants upon ANXA11 stability against aggregation, and differential muscle or motor neuron dysfunction expressed as a temporal and tissue-specific continuum.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Xavier Salvatella is a founder and board member of Nuage Therapeutics. The other authors report no disclosures.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
