Pd-Based Hybrid Nanoparticles As Multimodal Theranostic Nanomedicine
- PMID: 36651801
- PMCID: PMC9945085
- DOI: 10.1021/acsabm.2c00759
Pd-Based Hybrid Nanoparticles As Multimodal Theranostic Nanomedicine
Abstract
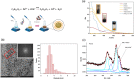
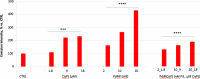
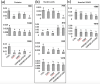
A nanodelivery system based on palladium nanoparticles (PdNP) and cisplatin (CisPt) was developed by physisorption of the drug onto the PdNP synthesized via a green redox process, using d-glucose and polyvinylpyrrolidone (PVP) as reducing and stabilizing/capping agents, respectively. UV-vis analysis and H2-evolution measurements were carried out to prove the nanoparticles' capability to act as bimodal theranostic nanomedicine, i.e., having both plasmonic and photocatalytic properties. XPS, XRD, and TEM allowed light to be shed on the chemical composition and morphology of the PdNP. The analysis of the UV-visible spectra evidenced plasmonic peak changes for the hybrid nanoparticle-drug assembly (Pd@CisPt), which pointed to a significant interaction of CisPt with the NP surface. The drug loading was quantitatively estimated by ICP-OES measurements, while DLS and AFM confirmed the strong association of the drug with the nanoparticle surface. The test of SOD-like activity in a cell-free environment proved the maintenance of the antioxidant capability of PdNP also in the Pd@CisPt systems. Finally, Pd@CisPt tested in prostate cancer cells (PC-3 line) unveiled the antitumoral action of the developed nanomedicine, related to reactive oxygen species (ROS) generation, with a condition of protein misfolding/unfolding and DNA damage, as evidenced by cytotoxicity and MitoSOX assays, as well as Raman microspectroscopy, respectively. Cell imaging by confocal microscopy evidenced cellular uptake of the nanoparticles, as well as dynamic processes of copper ion accumulation at the level of subcellular compartments. Finally, cell migration studies upon treatment with Pd@CisPt evidenced a tunable response between the inhibitory effect of CisPt and the enhanced rate of cell migration for the metal NP alone, which pointed out the promising potential of the developed theranostic nanomedicine in tissue regeneration.
Keywords: multimodal platform; photocatalytic activity; plasmonics; prostate cancer cell targeting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Sultan M. H.; Moni S. S.; Madkhali O. A.; Bakkari M. A.; Alshahrani S.; Alqahtani S. S.; Alhakamy N. A.; Mohan S.; Ghazwani M.; Bukhary H. A.; et al.Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468, 10.1038/s41598-021-04427-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

