A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia
- PMID: 36652019
- PMCID: PMC9849194
- DOI: 10.1007/s00018-022-04648-w
A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia
Abstract
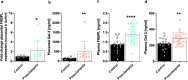
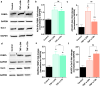
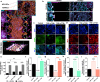
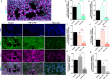
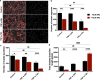
Preeclampsia is a pregnancy-specific cardiovascular disorder, involving significant maternal endothelial dysfunction. Although inappropriate placentation due to aberrant angiogenesis, inflammation and shallow trophoblast invasion are the root causes of preeclampsia, pathogenic mechanisms are poorly understood, particularly in early pregnancy. Here, we first confirm the abnormal expression of important vascular and inflammatory proteins, FK506-binding protein-like (FKBPL) and galectin-3 (Gal-3), in human plasma and placental tissues from women with preeclampsia and normotensive controls. We then employ a three-dimensional microfluidic placental model incorporating human umbilical vein endothelial cells (HUVECs) and a first trimester trophoblast cell line (ACH-3P) to investigate FKBPL and Gal-3 signaling in inflammatory conditions. In human samples, both circulating (n = 17 controls; n = 30 preeclampsia) and placental (n ≥ 6) FKBPL and Gal-3 levels were increased in preeclampsia compared to controls (plasma: FKBPL, p < 0.0001; Gal-3, p < 0.01; placenta: FKBPL, p < 0.05; Gal-3, p < 0.01), indicative of vascular dysfunction in preeclampsia. In our placenta-on-a-chip model, we show that endothelial cells are critical for trophoblast-mediated migration and that trophoblasts effectively remodel endothelial vascular networks. Inflammatory cytokine tumour necrosis factor-α (10 ng/mL) modulates both FKBPL and Gal-3 signaling in conjunction with trophoblast migration and impairs vascular network formation (p < 0.005). Our placenta-on-a-chip recapitulates aspects of inappropriate placental development and vascular dysfunction in preeclampsia.
Keywords: FKBPL; Galectin-3; Microfluidics; Placental development; Preeclampsia; Vascular remodeling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no financial conflict of interest. LM is an inventor on FKBPL-related patents.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

