Time-Action Profile of Technosphere Insulin in Children with Type 1 Diabetes
- PMID: 36652106
- PMCID: PMC9981820
- DOI: 10.1007/s13300-023-01368-7
Time-Action Profile of Technosphere Insulin in Children with Type 1 Diabetes
Abstract
Introduction: Technosphere insulin (TI) is an inhaled dry powder ultra-rapid-acting insulin. This report describes the results of the first study of TI in children with type 1 diabetes (T1D).
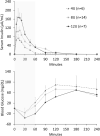
Methods: Pharmacokinetics (PK) of TI and the effect of TI on circulating glucose concentrations were evaluated in a single-arm study that enrolled children ages 8-17 years with T1D for more than 1 year, on a stable multiple daily insulin injection (MDI) regimen, and meeting pre-defined pulmonary function testing criteria (at least 70% predicted). To assess PK, subjects received an individualized single preprandial dose of TI (4-12 U, in 4-U increments) via oral inhalation, based on their usual meal-time subcutaneously injected rapid-acting insulin dose and meal content. Serum insulin and blood glucose were measured at - 30 to 250 min relative to dosing.
Results: Twenty-seven children with T1D participated in this single-dose PK study. Mean subject age was 13.3 years (59% female; 81.5% White). Mean serum insulin Cmax (maximum concentration) was 77.3, 119.15, and 207.7 µU/mL for doses of 4, 8, and 12 U, respectively. Tmax occurred at 10.5, 13.9, and 14.6 min post-dose for 4, 8, and 12 U. Glucose lowering 30-60 min post-dose was consistent with the PK profile.
Conclusion: Serum insulin rapidly increased post-dose and returned to baseline by 120 min. The data suggests the PK of TI in youth with T1D ages 8-17 years was similar to that seen in previous adult studies.
Trial registration: ClinicalTrials.gov identifier, NCT02527265.
Keywords: Inhaled insulin; Pediatric; Pharmacokinetics; Postprandial glucose; Type 1 diabetes.
© 2023. The Author(s).
Figures
References
-
- Fiasp Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208751s000lbl.pdf. Accessed 2 Jan 2022.
-
- Lyumjev Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761109s000lbl.pdf. Accessed 2 Jan 2022.
-
- Lispro Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020563s115lbl.pdf. Accessed 2 Jan 2022.
Associated data
LinkOut - more resources
Full Text Sources
Medical