Aspirin in diabetic patients at primary prevention: insights of the VITAL cohort
- PMID: 36652191
- PMCID: PMC10261209
- DOI: 10.1007/s40618-022-02001-3
Aspirin in diabetic patients at primary prevention: insights of the VITAL cohort
Abstract
Purpose: Aspirin use among patients with diabetes in primary prevention is still a matter of debate. We aimed to evaluate the potential cardiovascular risk benefit of aspirin in primary prevention, using data from a contemporary cohort.
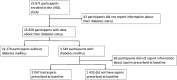
Methods: Retrospective analysis of the VITAL cohort with > 20,000 individuals at primary prevention who were followed for a median of 5.3 years. The population was evaluated according to the baseline diabetes status, and then aspirin use was evaluated among diabetic patients. Cox regression models were used to estimate the risks of mortality and cardiovascular outcomes. The estimates were reported using adjusted hazard ratio (HR) and 95% confidence intervals (95%CI).
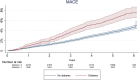
Results: Diabetic patients (n = 3549; 13.7%) showed to increase the risk of all-cause mortality (HR 1.61, 95%CI 1.33-1.94), and major adverse cardiovascular events (MACE) (HR 1.36 95%CI 1.11-1.68) than non-diabetic population. Diabetic patients taking aspirin were older, more frequently man, hypertensive, current users of statins, and current smokers compared with diabetic patients who did not use aspirin at baseline. There was no difference between diabetic aspirin users and non-users regarding all-cause mortality (HR 0.80, 95%CI 0.59, 1.10), MACE (HR 0.92, 95%CI 0.64, 1.33), coronary heart disease (HR 0.98, 95%CI 0.67, 1.43), or stroke (HR 0.87, 95%CI 0.48, 1.58).
Conclusions: The VITAL data confirmed diabetes as an important risk factor for cardiovascular events in a contemporary cohort but did not show cardiovascular benefits of aspirin in primary prevention among people with diabetes who were shown to be at higher risk of cardiovascular events.
Keywords: Aspirin; Cardiovascular disease; Diabetes; Primary prevention.
© 2023. The Author(s).
Conflict of interest statement
DC has participated in educational meetings and/or attended a conferences or symposia (including travel, accommodation and/or hospitality) with Bial, Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck Serono, Ferrer, Pfizer, Novartis and Roche. JJF is a consultant for Ipsen, GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, BIAL, Merck Serono, and Merz and received grants from GlaxoSmithKline, Grunenthal, Teva, and Fundação MSD. FJP had consultant and speaker fees with Astra Zeneca, Bayer, BMS, Boehringer Ingelheim and Daiichi Sankyo. The remaining authors have nothing to declare.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

