Trends in the Prevalence of Methylchloroisothiazolinone/Methylisothiazolinone Contact Allergy in North America and Europe
- PMID: 36652228
- PMCID: PMC9857829
- DOI: 10.1001/jamadermatol.2022.5991
Trends in the Prevalence of Methylchloroisothiazolinone/Methylisothiazolinone Contact Allergy in North America and Europe
Abstract
Importance: The common use of isothiazolinones as preservatives is a global cause of allergic contact dermatitis. Differences in allowable concentrations of methylisothiazolinone (MI) exist in Europe, Canada, and the US.
Objective: To compare the prevalence of positive patch test reactions to the methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) combination and MI alone in North America and Europe from 2009 to 2018.
Design, setting, and participants: This retrospective analysis of North American Contact Dermatitis Group, European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) databases included data from patients presenting for patch testing at referral patch test clinics in North America and Europe.
Exposures: Patch tests to MCI/MI and MI.
Main outcomes and measures: Prevalence of allergic contact dermatitis to MCI/MI and MI.
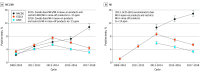
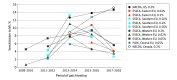
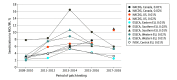
Results: From 2009 to 2018, participating sites in North America and Europe patch tested a total of 226 161 individuals to MCI/MI and 118 779 to MI. In Europe, positivity to MCI/MI peaked during 2013 and 2014 at 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) during 2017 and 2018. Positive reactions to MI were 5.5% (ESSCA) and 3.4% (IVDK) during 2017 and 2018. In North America, the frequency of positivity to MCI/MI increased steadily through the study period, reaching 10.8% for MCI/MI during 2017 and 2018. Positive reactions to MI were 15.0% during 2017 and 2018.
Conclusions and relevance: The study results suggest that in contrast to the continued increase in North America, isothiazolinone allergy is decreasing in Europe. This trend may coincide with earlier and more stringent government regulation of MI in Europe.
Conflict of interest statement
Figures