Persistent COVID-19 Symptoms at 6 Months After Onset and the Role of Vaccination Before or After SARS-CoV-2 Infection
- PMID: 36652247
- PMCID: PMC9857077
- DOI: 10.1001/jamanetworkopen.2022.51360
Persistent COVID-19 Symptoms at 6 Months After Onset and the Role of Vaccination Before or After SARS-CoV-2 Infection
Erratum in
-
Error in Figure 2B.JAMA Netw Open. 2023 Feb 1;6(2):e230734. doi: 10.1001/jamanetworkopen.2023.0734. JAMA Netw Open. 2023. PMID: 36757701 Free PMC article. No abstract available.
Abstract
Importance: Understanding the factors associated with post-COVID conditions is important for prevention.
Objective: To identify characteristics associated with persistent post-COVID-19 symptoms and to describe post-COVID-19 medical encounters.
Design, setting, and participants: This cohort study used data from the Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases With Pandemic Potential (EPICC) study implemented in the US military health system (MHS); MHS beneficiaries aged 18 years or older who tested positive for SARS-CoV-2 from February 28, 2020, through December 31, 2021, were analyzed, with 1-year follow-up.
Exposures: SARS-CoV-2 infection.
Main outcomes and measures: The outcomes analyzed included survey-reported symptoms through 6 months after SARS-CoV-2 infection and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis categories reported in medical records 6 months following SARS-CoV-2 infection vs 3 months before infection.
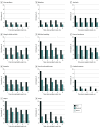
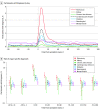
Results: More than half of the 1832 participants in these analyses were aged 18 to 44 years (1226 [66.9%]; mean [SD] age, 40.5 [13.7] years), were male (1118 [61.0%]), were unvaccinated at the time of their infection (1413 [77.1%]), and had no comorbidities (1290 [70.4%]). A total of 728 participants (39.7%) had illness that lasted 28 days or longer (28-89 days: 364 [19.9%]; ≥90 days: 364 [19.9%]). Participants who were unvaccinated prior to infection (risk ratio [RR], 1.39; 95% CI, 1.04-1.85), reported moderate (RR, 1.80; 95% CI, 1.47-2.22) or severe (RR, 2.25; 95% CI, 1.80-2.81) initial illnesses, had more hospitalized days (RR per each day of hospitalization, 1.02; 95% CI, 1.00-1.03), and had a Charlson Comorbidity Index score of 5 or greater (RR, 1.55; 95% CI, 1.01-2.37) were more likely to report 28 or more days of symptoms. Among unvaccinated participants, postinfection vaccination was associated with a 41% lower risk of reporting symptoms at 6 months (RR, 0.59; 95% CI, 0.40-0.89). Participants had higher risk of pulmonary (RR, 2.00; 95% CI, 1.40-2.84), diabetes (RR, 1.46; 95% CI, 1.00-2.13), neurological (RR, 1.29; 95% CI, 1.02-1.64), and mental health-related medical encounters (RR, 1.28; 95% CI, 1.01-1.62) at 6 months after symptom onset than at baseline (before SARS-CoV-2 infection).
Conclusions and relevance: In this cohort study, more severe acute illness, a higher Charlson Comorbidity Index score, and being unvaccinated were associated with a higher risk of reporting COVID-19 symptoms lasting 28 days or more. Participants with COVID-19 were more likely to seek medical care for diabetes, pulmonary, neurological, and mental health-related illness for at least 6 months after onset compared with their pre-COVID baseline health care use patterns. These findings may inform the risk-benefit ratio of COVID-19 vaccination policy.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

