Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years
- PMID: 36652353
- PMCID: PMC9933930
- DOI: 10.1056/NEJMoa2213082
Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years
Abstract
Background: The emergence of immune-escape variants of severe acute respiratory syndrome coronavirus 2 warrants the use of sequence-adapted vaccines to provide protection against coronavirus disease 2019.
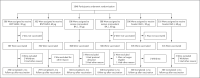
Methods: In an ongoing phase 3 trial, adults older than 55 years who had previously received three 30-μg doses of the BNT162b2 vaccine were randomly assigned to receive 30 μg or 60 μg of BNT162b2, 30 μg or 60 μg of monovalent B.1.1.529 (omicron) BA.1-adapted BNT162b2 (monovalent BA.1), or 30 μg (15 μg of BNT162b2 + 15 μg of monovalent BA.1) or 60 μg (30 μg of BNT162b2 + 30 μg of monovalent BA.1) of BA.1-adapted BNT162b2 (bivalent BA.1). Primary objectives were to determine superiority (with respect to 50% neutralizing titer [NT50] against BA.1) and noninferiority (with respect to seroresponse) of the BA.1-adapted vaccines to BNT162b2 (30 μg). A secondary objective was to determine noninferiority of bivalent BA.1 to BNT162b2 (30 μg) with respect to neutralizing activity against the ancestral strain. Exploratory analyses assessed immune responses against omicron BA.4, BA.5, and BA.2.75 subvariants.
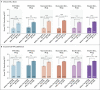
Results: A total of 1846 participants underwent randomization. At 1 month after vaccination, bivalent BA.1 (30 μg and 60 μg) and monovalent BA.1 (60 μg) showed neutralizing activity against BA.1 superior to that of BNT162b2 (30 μg), with NT50 geometric mean ratios (GMRs) of 1.56 (95% confidence interval [CI], 1.17 to 2.08), 1.97 (95% CI, 1.45 to 2.68), and 3.15 (95% CI, 2.38 to 4.16), respectively. Bivalent BA.1 (both doses) and monovalent BA.1 (60 μg) were also noninferior to BNT162b2 (30 μg) with respect to seroresponse against BA.1; between-group differences ranged from 10.9 to 29.1 percentage points. Bivalent BA.1 (either dose) was noninferior to BNT162b2 (30 μg) with respect to neutralizing activity against the ancestral strain, with NT50 GMRs of 0.99 (95% CI, 0.82 to 1.20) and 1.30 (95% CI, 1.07 to 1.58), respectively. BA.4-BA.5 and BA.2.75 neutralizing titers were numerically higher with 30-μg bivalent BA.1 than with 30-μg BNT162b2. The safety profile of either dose of monovalent or bivalent BA.1 was similar to that of BNT162b2 (30 μg). Adverse events were more common in the 30-μg monovalent-BA.1 (8.5%) and 60-μg bivalent-BA.1 (10.4%) groups than in the other groups (3.6 to 6.6%).
Conclusions: The candidate monovalent or bivalent omicron BA.1-adapted vaccines had a safety profile similar to that of BNT162b2 (30 μg), induced substantial neutralizing responses against ancestral and omicron BA.1 strains, and, to a lesser extent, neutralized BA.4, BA.5, and BA.2.75 strains. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04955626.).
Copyright © 2023 Massachusetts Medical Society.
Figures

References
-
- Pfizer–BioNTech. Highlights of prescribing information: comirnaty (COVID-19 vaccine, mRNA) (https://www.fda.gov/media/151707/download).
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
