Inhibition of autophagy in microglia and macrophages exacerbates innate immune responses and worsens brain injury outcomes
- PMID: 36652438
- PMCID: PMC10283445
- DOI: 10.1080/15548627.2023.2167689
Inhibition of autophagy in microglia and macrophages exacerbates innate immune responses and worsens brain injury outcomes
Abstract
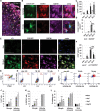
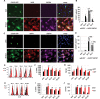
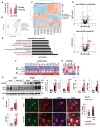
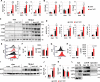
Excessive and prolonged neuroinflammation following traumatic brain injury (TBI) contributes to long-term tissue damage and poor functional outcomes. However, the mechanisms contributing to exacerbated inflammatory responses after brain injury remain poorly understood. Our previous work showed that macroautophagy/autophagy flux is inhibited in neurons following TBI in mice and contributes to neuronal cell death. In the present study, we demonstrate that autophagy is also inhibited in activated microglia and infiltrating macrophages, and that this potentiates injury-induced neuroinflammatory responses. Macrophage/microglia-specific knockout of the essential autophagy gene Becn1 led to overall increase in neuroinflammation after TBI. In particular, we observed excessive activation of the innate immune responses, including both the type-I interferon and inflammasome pathways. Defects in microglial and macrophage autophagy following injury were associated with decreased phagocytic clearance of danger/damage-associated molecular patterns (DAMP) responsible for activation of the cellular innate immune responses. Our data also demonstrated a role for precision autophagy in targeting and degradation of innate immune pathways components, such as the NLRP3 inflammasome. Finally, inhibition of microglial/macrophage autophagy led to increased neurodegeneration and worse long-term cognitive outcomes after TBI. Conversely, increasing autophagy by treatment with rapamycin decreased inflammation and improved outcomes in wild-type mice after TBI. Overall, our work demonstrates that inhibition of autophagy in microglia and infiltrating macrophages contributes to excessive neuroinflammation following brain injury and in the long term may prevent resolution of inflammation and tissue regeneration.Abbreviations: Becn1/BECN1, beclin 1, autophagy related; CCI, controlled cortical impact; Cybb/CYBB/NOX2: cytochrome b-245, beta polypeptide; DAMP, danger/damage-associated molecular patterns; Il1b/IL1B/Il-1β, interleukin 1 beta; LAP, LC3-associated phagocytosis; Map1lc3b/MAP1LC3/LC3, microtubule-associated protein 1 light chain 3 beta; Mefv/MEFV/TRIM20: Mediterranean fever; Nos2/NOS2/iNOS: nitric oxide synthase 2, inducible; Nlrp3/NLRP3, NLR family, pyrin domain containing 3; Sqstm1/SQSTM1/p62, sequestosome 1; TBI, traumatic brain injury; Tnf/TNF/TNF-α, tumor necrosis factor; Ulk1/ULK1, unc-51 like kinase 1.
Keywords: Autophagy; innate immunity; macrophage; microglia; neuroinflammation; traumatic brain injury.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
