A Phase I Dose-escalation Study of AZD3965, an Oral Monocarboxylate Transporter 1 Inhibitor, in Patients with Advanced Cancer
- PMID: 36652553
- PMCID: PMC7614436
- DOI: 10.1158/1078-0432.CCR-22-2263
A Phase I Dose-escalation Study of AZD3965, an Oral Monocarboxylate Transporter 1 Inhibitor, in Patients with Advanced Cancer
Abstract
Purpose: Inhibition of monocarboxylate transporter (MCT) 1-mediated lactate transport may have cytostatic and/or cytotoxic effects on tumor cells. We report results from the dose-escalation part of a first-in-human trial of AZD3965, a first-in-class MCT1 inhibitor, in advanced cancer.
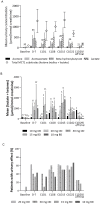
Patients and methods: This multicentre, phase I, dose-escalation and dose-expansion trial enrolled patients with advanced solid tumors or lymphoma and no standard therapy options. Exclusion criteria included history of retinal and/or cardiac disease, due to MCT1 expression in the eye and heart. Patients received daily oral AZD3965 according to a 3+3 then rolling six design. Primary objectives were to assess safety and determine the MTD and/or recommended phase II dose (RP2D). Secondary objectives for dose escalation included measurement of pharmacokinetic and pharmacodynamic activity. Exploratory biomarkers included tumor expression of MCT1 and MCT4, functional imaging of biological impact, and metabolomics.
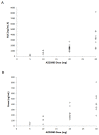
Results: During dose escalation, 40 patients received AZD3965 at 5-30 mg once daily or 10 or 15 mg twice daily. Treatment-emergent adverse events were primarily grade 1 and/or 2, most commonly electroretinogram changes (retinopathy), fatigue, anorexia, and constipation. Seven patients receiving ≥20 mg daily experienced dose-limiting toxicities (DLT): grade 3 cardiac troponin rise (n = 1), asymptomatic ocular DLTs (n = 5), and grade 3 acidosis (n = 1). Plasma pharmacokinetics demonstrated attainment of target concentrations; pharmacodynamic measurements indicated on-target activity.
Conclusions: AZD3965 is tolerated at doses that produce target engagement. DLTs were on-target and primarily dose-dependent, asymptomatic, reversible ocular changes. An RP2D of 10 mg twice daily was established for use in dose expansion in cancers that generally express high MCT1/low MCT4).
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:113. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Chesney J, Telang S. Regulation of glycolytic and mitochondrial metabolism by ras. Curr Pharm Biotechnol. 2013;14:251–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

