Circulating uridine dynamically and adaptively regulates food intake in humans
- PMID: 36652907
- PMCID: PMC9873946
- DOI: 10.1016/j.xcrm.2022.100897
Circulating uridine dynamically and adaptively regulates food intake in humans
Abstract
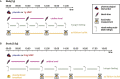
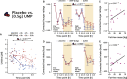
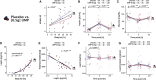
Feeding behavior must be continuously adjusted to match energy needs. Recent discoveries in murine models identified uridine as a regulator of energy balance. Here, we explore its contribution to the complex control of food intake in humans by administering a single dose of uridine monophosphate (UMP; 0.5 or 1 g) to healthy participants in two placebo-controlled studies designed to assess food behavior (registration: DRKS00014874). We establish that endogenous circulating uridine correlates with hunger and ensuing food consumption. It also dynamically decreases upon caloric ingestion, prompting its potential role in a negative feedback loop regulating energy intake. We further demonstrate that oral UMP administration temporarily increases circulating uridine and-when within the physiological range-enhances hunger and caloric intake proportionally to participants' basal energy needs. Overall, uridine appears as a potential target to tackle dysfunctions of feeding behavior in humans.
Keywords: adaptive behavior; food intake; hunger; uridine.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests O.A.C. reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis; consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, and Pfizer; payment for expert testimony from Cidara; participation on a data safety monitoring board or advisory board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, and Shionogi; a pending patent from the German Patent and Trade Mark Office; and other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, and Wiley.
Figures



Comment in
-
A role for circulating levels of uridine in regulating hunger and food intake.Nat Rev Endocrinol. 2023 Apr;19(4):188. doi: 10.1038/s41574-023-00808-5. Nat Rev Endocrinol. 2023. PMID: 36732645 No abstract available.
References
-
- Karle J.M., Anderson L.W., Erlichman C., Cysyk R.L. Serum uridine levels in patients receiving N-(phosphonacetyl)-L-aspartate. Cancer Res. 1980;40:2938–2940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

