Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population
- PMID: 36652990
- PMCID: PMC9941049
- DOI: 10.1038/s41591-023-02219-5
Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population
Erratum in
-
Author Correction: Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population.Nat Med. 2024 Jan;30(1):305. doi: 10.1038/s41591-023-02648-2. Nat Med. 2024. PMID: 37875568 Free PMC article. No abstract available.
Abstract
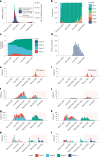
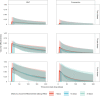
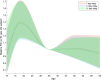
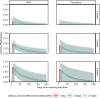
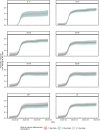
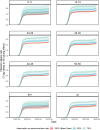
The SARS-CoV-2 Omicron variant has demonstrated enhanced transmissibility and escape of vaccine-derived immunity. Although first-generation vaccines remain effective against severe disease and death, robust evidence on vaccine effectiveness (VE) against all Omicron infections, irrespective of symptoms, remains sparse. We used a community-wide serosurvey with 5,310 subjects to estimate how vaccination histories modulated risk of infection in infection-naive Hong Kong during a large wave of Omicron BA.2 epidemic in January-July 2022. We estimated that Omicron infected 45% (41-48%) of the local population. Three and four doses of BNT162b2 or CoronaVac were effective against Omicron infection 7 days after vaccination (VE of 48% (95% credible interval 34-64%) and 69% (46-98%) for three and four doses of BNT162b2, respectively; VE of 30% (1-66%) and 56% (6-97%) for three and four doses of CoronaVac, respectively). At 100 days after immunization, VE waned to 26% (7-41%) and 35% (10-71%) for three and four doses of BNT162b2, and to 6% (0-29%) and 11% (0-54%) for three and four doses of CoronaVac. The rapid waning of VE against infection conferred by first-generation vaccines and an increasingly complex viral evolutionary landscape highlight the necessity for rapidly deploying updated vaccines followed by vigilant monitoring of VE.
© 2023. The Author(s).
Conflict of interest statement
A.H., M.P. and S.A.V. have filed an IDF (US 63/016,898) for the use of ORF8 and ORF3b as diagnostics of SARS-CoV-2 infection. M.M. produced ORF8 by patent process based on US Patents 8,507,220 and 8,586,826. The other authors declare no competing interests.
Figures








References
-
- Statistics on 5th Wave of COVID-19 (from 31 Dec 2021 up till 31 Jul 2022 00:00) (The Government of the Hong Kong Special Administrative Region, 2022); https://www.coronavirus.gov.hk/pdf/5th_wave_statistics/5th_wave_statisti...
-
- Modelling the Fifth Wave of COVID-19 in Hong Kong – Update #9 (The Univ. of Hong Kong, 2022); https://www.med.hku.hk/en/news/press//-/media/HKU-Med-Fac/News/slides/20...
-
- Amman F, et al. Viral variant-resolved wastewater surveillance of SARS-CoV-2 at national scale. Nat. Biotechnol. 2022;40:1814–1822. - PubMed
-
- Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

