Characterising the autoantibody repertoire in systemic sclerosis following myeloablative haematopoietic stem cell transplantation
- PMID: 36653124
- PMCID: PMC10176357
- DOI: 10.1136/ard-2021-221926
Characterising the autoantibody repertoire in systemic sclerosis following myeloablative haematopoietic stem cell transplantation
Abstract
Objectives: Results from the SCOT (Scleroderma: Cyclophosphamide Or Transplantation) clinical trial demonstrated significant benefits of haematopoietic stem cell transplant (HSCT) versus cyclophosphamide (CTX) in patients with systemic sclerosis. The objective of this study was to test the hypothesis that transplantation stabilises the autoantibody repertoire in patients with favourable clinical outcomes.
Methods: We used a bead-based array containing 221 protein antigens to profile serum IgG autoantibodies in participants of the SCOT trial.
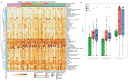
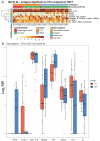
Results: Comparison of autoantibody profiles at month 26 (n=23 HSCT; n=22 CTX) revealed antibodies against two viral antigens and six self-proteins (SSB/La, CX3CL1, glycyl-tRNA synthetase (EJ), parietal cell antigen, bactericidal permeability-increasing protein and epidermal growth factor receptor (EGFR)) that were significantly different between treatment groups. Linear mixed model analysis identified temporal increases in antibody levels for hepatitis B surface antigen, CCL3 and EGFR in HSCT-treated patients. Eight of 32 HSCT-treated participants and one of 31 CTX-treated participants had temporally varying serum antibody profiles for one or more of 14 antigens. Baseline autoantibody levels against 20 unique antigens, including 9 secreted proteins (interleukins, IL-18, IL-22, IL-23 and IL-27), interferon-α2A, stem cell factor, transforming growth factor-β, macrophage colony-stimulating factor and macrophage migration inhibitory factor were significantly higher in patients who survived event-free to month 54.
Conclusions: Our results suggest that HSCT favourably alters the autoantibody repertoire, which remains virtually unchanged in CTX-treated patients. Although antibodies recognising secreted proteins are generally thought to be pathogenic, our results suggest a subset could potentially modulate HSCT in scleroderma.
Keywords: Autoantibodies; Scleroderma, Systemic; Systemic Sclerosis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LC serves on the advisory board or received consulting fees from Mitsubishi Tanabe, Jannsen, Genentech, Kyverna, and Eicos Sciences.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- HHSN272201100025C/AI/NIAID NIH HHS/United States
- P30 AR073750/AR/NIAMS NIH HHS/United States
- U19 AI110491/AI/NIAID NIH HHS/United States
- U01 AI165527/AI/NIAID NIH HHS/United States
- R01 AI125197/AI/NIAID NIH HHS/United States
- HHSN272200900057C/AI/NIAID NIH HHS/United States
- UM1 AI144292/AI/NIAID NIH HHS/United States
- N01 AI025481/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- 75N93022C00052/AI/NIAID NIH HHS/United States
- U19 AI109662/AI/NIAID NIH HHS/United States
- N01 AI005419/AI/NIAID NIH HHS/United States
- U19 AI167903/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

