Evaluation of the host immune response assay SeptiCyte RAPID for potential triage of COVID-19 patients
- PMID: 36653401
- PMCID: PMC9845827
- DOI: 10.1038/s41598-023-28178-y
Evaluation of the host immune response assay SeptiCyte RAPID for potential triage of COVID-19 patients
Abstract
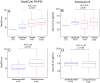
Tools for the evaluation of COVID-19 severity would help clinicians with triage decisions, especially the decision whether to admit to ICU. The aim of this study was to evaluate SeptiCyte RAPID, a host immune response assay (Immunexpress, Seattle USA) as a triaging tool for COVID-19 patients requiring hospitalization and potentially ICU care. SeptiCyte RAPID employs a host gene expression signature consisting of the ratio of expression levels of two immune related mRNAs, PLA2G7 and PLAC8, measured from whole blood samples. Blood samples from 146 adult SARS-CoV-2 (+) patients were collected within 48 h of hospital admission in PAXgene blood RNA tubes at Hospital del Mar, Barcelona, Spain, between July 28th and December 1st, 2020. Data on demographics, vital signs, clinical chemistry parameters, radiology, interventions, and SeptiCyte RAPID were collected and analyzed with bioinformatics methods. The performance of SeptiCyte RAPID for COVID-19 severity assessment and ICU admission was evaluated, relative to the comparator of retrospective clinical assessment by the Hospital del Mar clinical care team. In conclusion, SeptiCyte RAPID was able to stratify COVID-19 cases according to clinical severity: critical vs. mild (AUC = 0.93, p < 0.0001), critical vs. moderate (AUC = 0.77, p = 0.002), severe vs. mild (AUC = 0.85, p = 0.0003), severe vs. moderate (AUC = 0.63, p = 0.05). This discrimination was significantly better (by AUC or p-value) than could be achieved by CRP, lactate, creatine, IL-6, or D-dimer. Some of the critical or severe cases had "early" blood draws (before ICU admission; n = 33). For these cases, when compared to moderate and mild cases not in ICU (n = 37), SeptiCyte RAPID had AUC = 0.78 (p = 0.00012). In conclusion, SeptiCyte RAPID was able to stratify COVID-19 cases according to clinical severity as defined by the WHO COVID-19 Clinical Management Living Guidance of January 25th, 2021. Measurements taken early (before a patient is considered for ICU admission) suggest that high SeptiScores could aid in predicting the need for later ICU admission.
© 2023. The Author(s).
Conflict of interest statement
TDY, KN, JTK are employees and shareholders in Immunexpress. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

