The systemic and hepatic alternative renin-angiotensin system is activated in liver cirrhosis, linked to endothelial dysfunction and inflammation
- PMID: 36653504
- PMCID: PMC9849268
- DOI: 10.1038/s41598-023-28239-2
The systemic and hepatic alternative renin-angiotensin system is activated in liver cirrhosis, linked to endothelial dysfunction and inflammation
Abstract
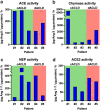
We aimed to assess the systemic and hepatic renin-angiotensin-system (RAS) fingerprint in advanced chronic liver disease (ACLD). This prospective study included 13 compensated (cACLD) and 12 decompensated ACLD (dACLD) patients undergoing hepatic venous pressure gradient (HVPG) measurement. Plasma components (all patients) and liver-local enzymes (n = 5) of the RAS were analyzed using liquid chromatography-tandem mass spectrometry. Patients with dACLD had significantly higher angiotensin (Ang) I, Ang II and aldosterone plasma levels. Ang 1-7, a major mediator of the alternative RAS, was almost exclusively detectable in dACLD (n = 12/13; vs. n = 1/13 in cACLD). Also, dACLD patients had higher Ang 1-5 (33.5 pmol/L versus cACLD: 6.6 pmol/L, p < 0.001) and numerically higher Ang III and Ang IV levels. Ang 1-7 correlated with HVPG (ρ = 0.655; p < 0.001), von Willebrand Factor (ρ = 0.681; p < 0.001), MELD (ρ = 0.593; p = 0.002) and interleukin-6 (ρ = 0.418; p = 0.047). Considerable activity of ACE, chymase, ACE2, and neprilysin was detectable in all liver biopsies, with highest chymase and ACE2 activity in cACLD patients. While liver-local classical and alternative RAS activity was already observed in cACLD, systemic activation of alternative RAS components occurred only in dACLD. Increased Ang 1-7 was linked to severe liver disease, portal hypertension, endothelial dysfunction and inflammation.
© 2023. The Author(s).
Conflict of interest statement
The authors have nothing to disclose regarding the work under consideration for publication. Conflicts of interests outside the submitted work: L.H., B.R. M.J., R.P. have nothing to disclose. O.D. and M.P: are employed at Attoquant Diagnostics. B.Sim. received travel support from AbbVie and Gilead. D.B. received speaker fees from AbbVie and Siemens, as well as grant support form Gilead and Siemens, as well as travel support from AbbVie and Gilead. M.T. served as a speaker and/or consultant and/or advisory board member for Albireo, BiomX, Falk, Boehringer Ingelheim, Bristol-Myers Squibb, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, and Shire, and received travel support from AbbVie, Falk, Gilead, and Intercept, as well as grants/research support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx. He is also co-inventor of patents on the medical use of 24-norursodeoxycholic acid. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Gilead, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. M.H. served as a speaker and/or consultant for Astellas Pharma, AstraZeneca, Eli Lilly, Fresenius Medical Care, Janssen-Cilag, Siemens Healthcare and Vifor and received academic study support from Astellas Pharma, Boehringer Ingelheim, Eli Lilly, Nikkiso and Siemens Healthcare. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Pliant, Philips, Siemens, and W. L. Gore & Associates as well as travel support from Abbvie, Boehringer Ingelheim, Gilead and Roche.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

