Acclimatization of a coral-dinoflagellate mutualism at a CO2 vent
- PMID: 36653505
- PMCID: PMC9849335
- DOI: 10.1038/s42003-022-04327-3
Acclimatization of a coral-dinoflagellate mutualism at a CO2 vent
Abstract
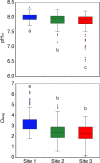
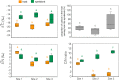
Ocean acidification caused by shifts in ocean carbonate chemistry resulting from increased atmospheric CO2 concentrations is threatening many calcifying organisms, including corals. Here we assessed autotrophy vs heterotrophy shifts in the Mediterranean zooxanthellate scleractinian coral Balanophyllia europaea acclimatized to low pH/high pCO2 conditions at a CO2 vent off Panarea Island (Italy). Dinoflagellate endosymbiont densities were higher at lowest pH Sites where changes in the distribution of distinct haplotypes of a host-specific symbiont species, Philozoon balanophyllum, were observed. An increase in symbiont C/N ratios was observed at low pH, likely as a result of increased C fixation by higher symbiont cell densities. δ13C values of the symbionts and host tissue reached similar values at the lowest pH Site, suggesting an increased influence of autotrophy with increasing acidification. Host tissue δ15N values of 0‰ strongly suggest that diazotroph N2 fixation is occurring within the coral tissue/mucus at the low pH Sites, likely explaining the decrease in host tissue C/N ratios with acidification. Overall, our findings show an acclimatization of this coral-dinoflagellate mutualism through trophic adjustment and symbiont haplotype differences with increasing acidification, highlighting that some corals are capable of acclimatizing to ocean acidification predicted under end-of-century scenarios.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Keys PW, et al. Anthropocene risk. Nat. Sustain. 2019;2:667–673. doi: 10.1038/s41893-019-0327-x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

